Cross-Linking Procedure
A breakthrough procedure for corneal cross-linking
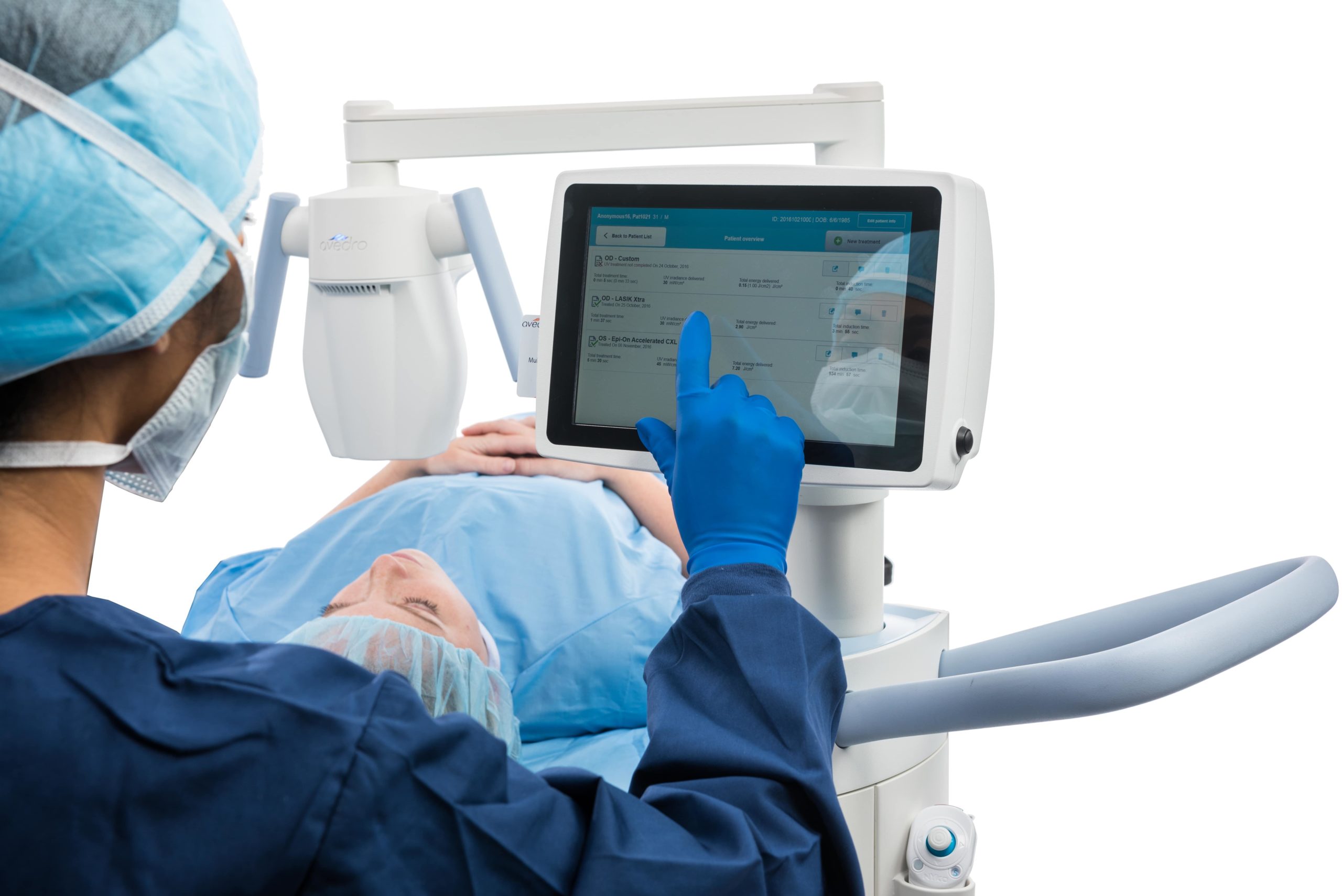
Using the latest generation KXL® system, our high-power cross-linking procedure has revolutionized traditional cross-linking by reducing treatment time to as little as a few minutes—all while maintaining a high standard of proven safety. The reduction in speed is made possible by increasing the ultraviolet light (UVA) power and reducing the exposure time, thereby maintaining the same energy on the eye as standard cross-linking while reducing cross-linking time by an order of magnitude.

Improved practice efficiency

The higher power output of our technology allows shorter UVA exposure times leading to improved patient experience, as well as improved practice efficiency.

How corneal cross-linking works
In the cross-linking procedure, riboflavin (vitamin B2) is dripped onto the cornea and then exposed to ultraviolet light. The light causes the riboflavin to fluoresce, which leads to the formation of bonds between collagen molecules called collagen cross-links. In a matter of minutes, the procedure is complete.
Who can benefit from corneal cross-linking?
Keratoconus patients:
Patients suffering from keratoconus experience a thinning of the cornea that causes visual impairments and could lead to corneal transplantation with progression of the condition. Cross-linking has the ability to stabilize the cornea, repairing its biomechanical integrity and halting or slowing the progression of keratoconus to preserve visual function.
Post-LASIK ectasia patients:
Corneal ectasia is a complication that can occur following a LASIK procedure and may result in increasing myopia and astigmatism, loss of visual acuity, steepening of the corneal curvature, and topographic asymmetric steepening of the inferior corneal curvature. Whether a patient develops post-LASIK ectasia weeks or even years after the procedure, the cross-linking procedure may be able to stabilize and strengthen the cornea.
We want to hear from you!
Please contact us to learn more about our cross-linking products and procedures.
Contact Us
"*" indicates required fields