Clinical Data
Halt the Progression of Keratoconus
Prospective clinical study conducted in the UK demonstrates the power of iLink® V with VibeX Rapid and the KXL to slow or halt the progression of keratoconus.1
Pre- and post-operative progression defined using the limits of keratometric repeatability established by the Global Delphi Panel of Keratoconus and Ectatic Diseases.2
Defining disease progression in early keratoconus and moderate to advanced keratoconus
- ≥ 1 D increase Kmax
- ≥ 1 D increase front K1 or K2
- ≥ 0.5 D increase back K2
- ≥ 16 μm decrease minimum corneal thickness
- ≥ 2.5 D increase Kmax
- ≥ 2.5 D increase front K1 or K2
- ≥ 22 μm decrease minimum corneal thickness
Criteria for determining progression in early and moderate/advanced keratoconus, based on differences in measurement repeatability limits in early and advanced keratoconus. Kmax, maximum keratometry; K1, flat keratometry in the central 3 mm zone; K2, steep keratometry in central 3 mm zone
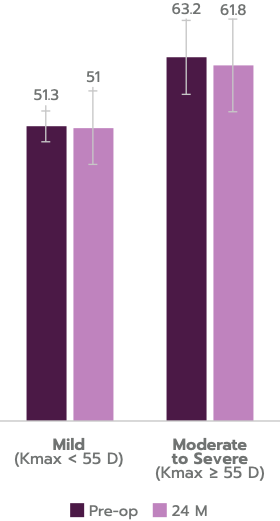
98.3% of progressive keratoconus patients treated with iLink® V achieved stability at 24 months.
No significant reduction in corrected distance visual acuity (CDVA), endothelial cell density (ECD), no clinical endothelial cell decompensation observed in this independent clinical study.
Short Procedure Duration with Long Term Benefits
Five-Year Outcomes of Accelerated Corneal Cross-linking using iLink® V
Independent retrospective study of accelerated cross-linking with iLink® V in patients with mild-to-moderate keratoconus (Amsler-Krumeich Grade I to III) demonstrated stability or improvement in Kmax between 24 months and 5 years.3
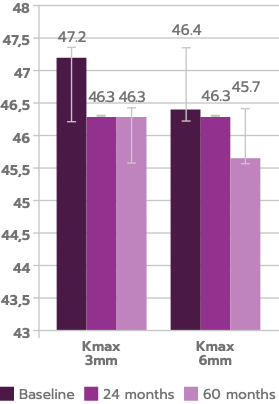
Anterior Kmax at 3 mm decreased significantly by 24 months (P = .009), remaining reduced relative to baseline at 60 months (P = .017), n=29.
Anterior Kmax at 6 mm decreased significantly by 24 months (P = .035), remaining reduced relative to baseline at 60 months (P = .027) n=29.
No postoperative complications such as corneal infections, infiltrates, or irreversible corneal opacities were observed at any time point during this study.
Citations:
- Gore, Daniel M., et al. American Journal of Ophthalmology 2021 Jan;221:9-16
- Gomes JA, Tan D, Rapuano CJ, Belin MW, Ambrósio R Jr, Guell JL, Malecaze F, Nishida K, Sangwan VS; Group of Panelists for the Global Delphi Panel of Keratoconus and Ectatic Diseases. Global consensus on keratoconus and ectatic diseases. Cornea. 2015
- Moramarco A, et al. J Refract Surg. 2020 Nov 1;36(11):724-730.
