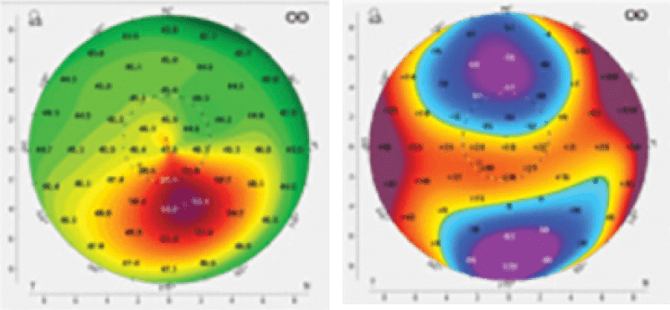
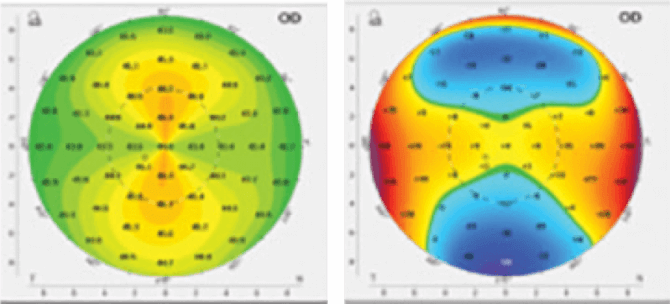
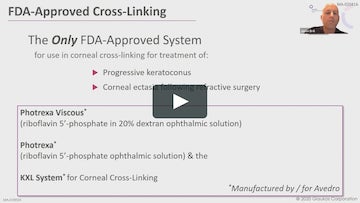
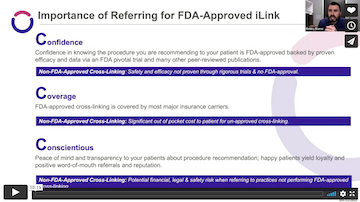
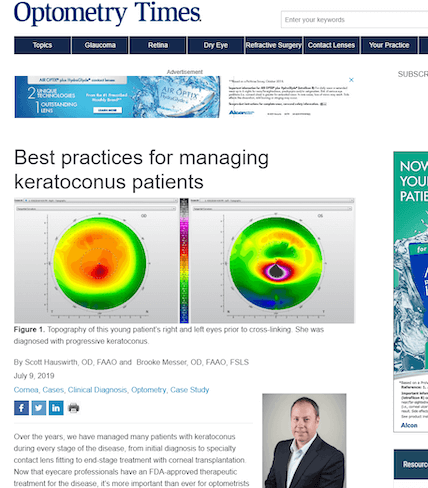
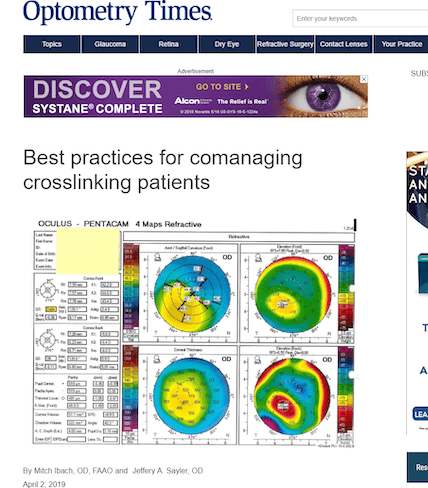
Using Photrexa® Viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution), Photrexa® (riboflavin 5’-phosphate ophthalmic solution), and the KXL® System, the iLink® corneal cross-linking procedure from Glaukos is the only FDA-approved therapeutic treatment for patients with progressive keratoconus and corneal ectasia following refractive surgery.*1
[Photrexa IFU/p1/col1/para3/lines1-4]
Important Safety Information
Corneal collagen cross-linking should not be performed on pregnant women.
Ulcerative keratitis can occur. Patients should be monitored for resolution of epithelial defects. The most common ocular adverse reaction was corneal opacity (haze). Other ocular side effects include punctate keratitis, corneal striae, dry eye, corneal epithelium defect, eye pain, light sensitivity, reduced visual acuity, and blurred vision.
These are not all the side effects of the corneal collagen cross-linking treatment. For more information, go to Prescribing Info to obtain the FDA-approved product labeling.
You are encouraged to report all side effects to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
*Photrexa® Viscous and Photrexa® are manufactured for Avedro. The KXL® System is manufactured by Avedro. Avedro is a wholly owned subsidiary of Glaukos Corporation.
Indications
Photrexa® Viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution) and Photrexa® (riboflavin 5’-phosphate ophthalmic solution) are indicated for use with the KXL System in corneal collagen cross-linking for the treatment of progressive keratoconus and corneal ectasia following refractive surgery.
Reference
1. Photrexa Prescribing Information. Burlington, MA: Avedro, a Glaukos company 2022