
Early onset: The vast majority of cases present between 12 and 20 years of age. Onset may also occur at birth and up to 51 years of age.2
iDetect KC
The iDetect KC program makes advanced topography more accessible than ever before. For a fraction of the price, optometrists can bring this essential diagnostic tool into their practice so they can:
Get more information about iDetect KC

Early onset: The vast majority of cases present between 12 and 20 years of age. Onset may also occur at birth and up to 51 years of age.2

Keratoconus is a rare and sight-threatening disease, US prevalence has been reported to be 1 in 2000.7
iDetect KC equips optometrists with advanced topography so they can implement the 3 pillars of modern keratoconus care into their practice.

A topographer can help optometrists:
Identify keratoconus
patients sooner
Monitor keratoconus progression
Preserve vision with
earlier intervention
Confidently refer patients to
an ophthalmologist for
treatment with iLink®

The iLink® corneal cross-linking procedure:
Slows or halts progressive keratoconus
Is proven safe and effective5
Is eligible for commercial
insurance coverage

Topography enables
optometrists to:
Provide long-term care for patients
Expand their contact lens service
With access to advanced topography, optometrists can have greater confidence in their assessments, a simplified diagnostic and treatment journey, and more control over the patient experience.
Bring advanced topography into your practice
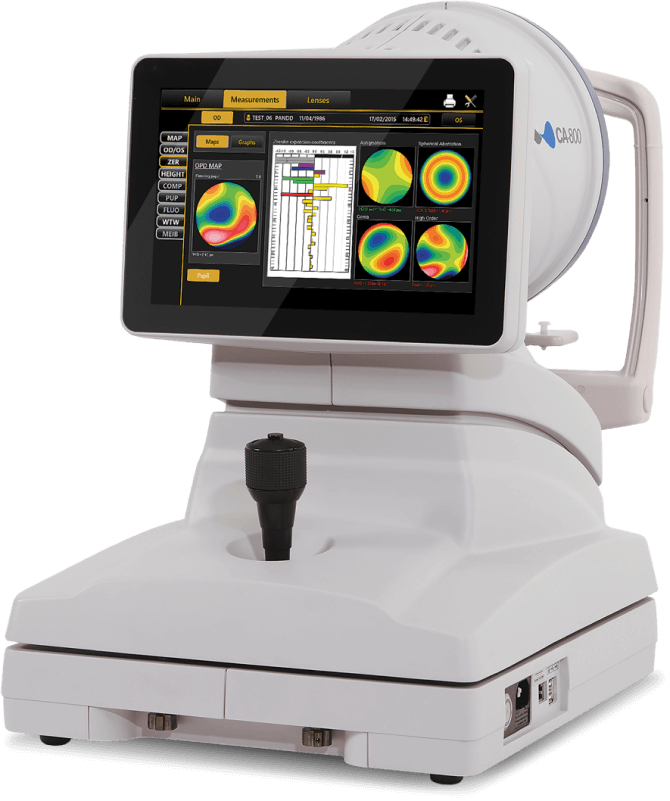
The iDetect KC Program
Glaukos and Topcon have partnered to offer optometrists the advanced technology of the Topcon CA-800 topographer at a lower cost of entry.
The iDetect KC data registry makes it easy to leave a lasting impact on the future of keratoconus care by uploading data from:
Complete the form below to find out more and we will be in touch with you shortly.
"*" indicates required fields
References
Using Photrexa® Viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution), Photrexa® (riboflavin 5’-phosphate ophthalmic solution), and the KXL® System, the iLink® corneal cross-linking procedure from Glaukos is the only FDA-approved therapeutic treatment for patients with progressive keratoconus and corneal ectasia following refractive surgery.*1
[Photrexa IFU/p1/col1/para3/lines1-4]
Corneal collagen cross-linking should not be performed on pregnant women.
Ulcerative keratitis can occur. Patients should be monitored for resolution of epithelial defects. The most common ocular adverse reaction was corneal opacity (haze). Other ocular side effects include punctate keratitis, corneal striae, dry eye, corneal epithelium defect, eye pain, light sensitivity, reduced visual acuity, and blurred vision.
These are not all the side effects of the corneal collagen cross-linking treatment. For more information, go to Prescribing Info to obtain the FDA-approved product labeling.
You are encouraged to report all side effects to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
*Photrexa® Viscous and Photrexa® are manufactured for Avedro. The KXL® System is manufactured by Avedro. Avedro is a wholly owned subsidiary of Glaukos Corporation.
Photrexa® Viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution) and Photrexa® (riboflavin 5’-phosphate ophthalmic solution) are indicated for use with the KXL System in corneal collagen cross-linking for the treatment of progressive keratoconus and corneal ectasia following refractive surgery.
1. Photrexa Prescribing Information. Burlington, MA: Avedro, a Glaukos company 2022