The medical necessity of iLink® corneal cross-linking has become widely recognized.
As a result, more than 95% of the commercially insured population has access to this procedure.
Reimbursement for Cross-Linking
The medical necessity of iLink® corneal cross-linking has become widely recognized.
As a result, more than 95% of the commercially insured population has access to this procedure.
The medical necessity of iLink® corneal cross-linking has become widely recognized. As a result, more than 95% of the commercially insured population has access to this procedure.
If you have questions about insurance policies for iLink® for the treatment of progressive keratoconus, please email your Market Access & Customer Analytics contact or reach out to marketaccess@glaukos.com.
iLink® is the only FDA-approved product for corneal cross-linking and the procedure is performed epi-off. Many insurance policies have detail about the fact that the epi-off procedure is the only FDA-approved treatment for progressive keratoconus that is being covered, while epi-on is not. See the examples below from Aetna, Cigna, and other insurance coverage policies.
“Aetna considers epithelium-off photochemical collagen cross-linkage using riboflavin and ultraviolet A medically necessary for keratoconus and keratectasia. Aetna considers epithelium-on (transepithelial) collagen cross-linkage experimental and investigational for keratoconus, keratectasia, and all other indications.”
“Conventional, epithelium-off, corneal collagen crosslinking (C-CXL) using a FDA-approved drug/device system (e.g., Photrexa® Viscous or Photrexa® with the KXL® System) (CPT Code® 0402T; HCPCS Code J3490) is considered medically necessary for the treatment of progressive keratoconus and corneal ectasia following refractive surgery.”
“Currently, the only corneal cross-linking treatment approved by the Food and Drug Administration (FDA) is the epithelium-off method. There are no FDA-approved corneal cross-linking treatments using the epithelium-on method.”
“Moda Health considers corneal collagen cross-linking (CXL) medically necessary if the requested procedure is for epithelium-off photochemical collagen cross-linkage using riboflavin (HCPC: J3490- Avedro’s Photrexa) and ultraviolet A.”
“SelectHealth covers epithelium-off corneal cross-linking once per lifetime if the following criteria are met: The medicine used is Photrexa® Viscous/Photrexa® with the KXL® device.”
Generally, insurance does not cover products and procedures that have not received FDA approval. As an example, the Blue Cross and Blue Shield Association’s Technology Evaluation Center (Premera BCBS Technology Review) states the following as part of their new technology evaluation criteria: “The technology must have final approval from the appropriate governmental regulatory bodies.”
The iPath360 is a full-service end-to-end program designed to assist patients who are seeking access and reimbursement for iLink® corneal cross-linking.
J-code J2787 has been issued by the Centers for Medicare and Medicaid Services for the use of Photrexa® Viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution) and Photrexa® (riboflavin 5’-phosphate ophthalmic solution) formulas.
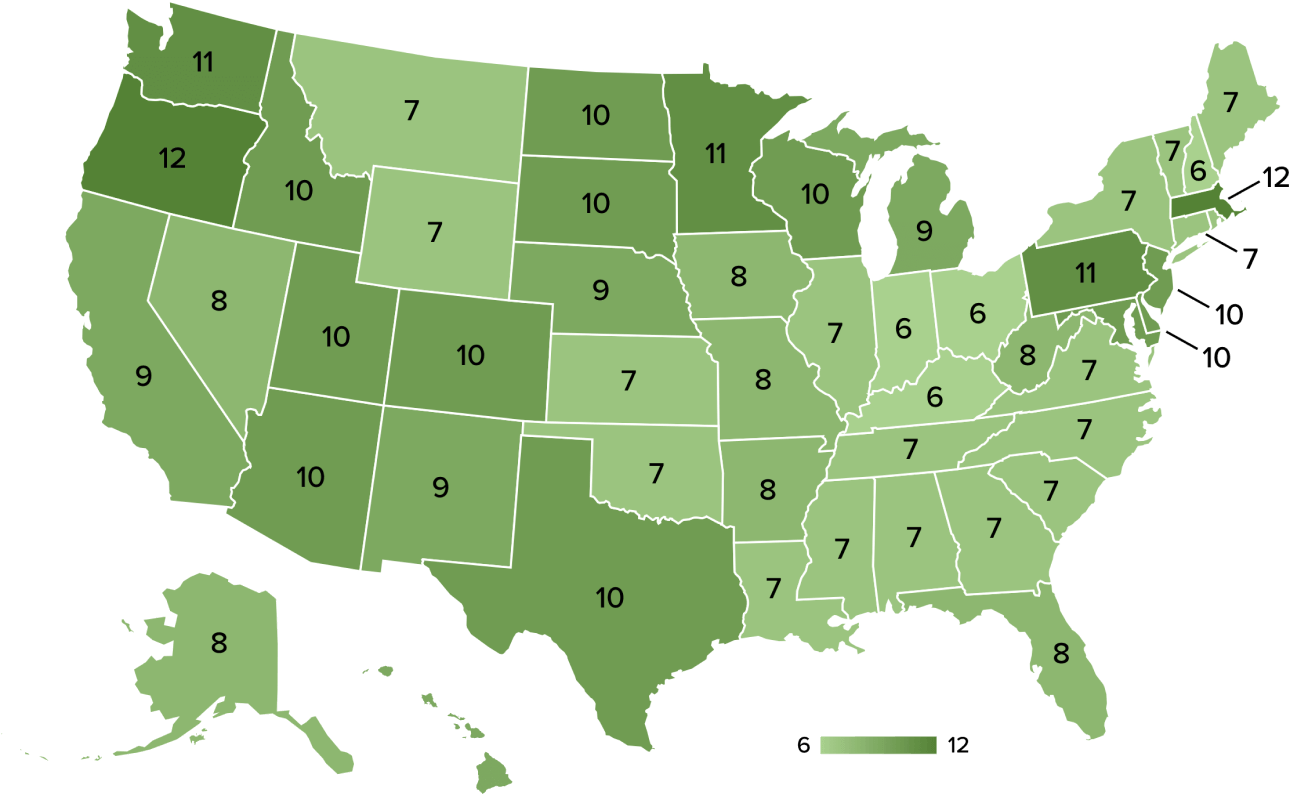
All 50 states have 6 or more plans that cover iLink® corneal cross-linking.
Using Photrexa® Viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution), Photrexa® (riboflavin 5’-phosphate ophthalmic solution), and the KXL® System, the iLink® corneal cross-linking procedure from Glaukos is the only FDA-approved therapeutic treatment for patients with progressive keratoconus and corneal ectasia following refractive surgery.*1
[Photrexa IFU/p1/col1/para3/lines1-4]
Corneal collagen cross-linking should not be performed on pregnant women.
Ulcerative keratitis can occur. Patients should be monitored for resolution of epithelial defects. The most common ocular adverse reaction was corneal opacity (haze). Other ocular side effects include punctate keratitis, corneal striae, dry eye, corneal epithelium defect, eye pain, light sensitivity, reduced visual acuity, and blurred vision.
These are not all the side effects of the corneal collagen cross-linking treatment. For more information, go to Prescribing Info to obtain the FDA-approved product labeling.
You are encouraged to report all side effects to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
*Photrexa® Viscous and Photrexa® are manufactured for Avedro. The KXL® System is manufactured by Avedro. Avedro is a wholly owned subsidiary of Glaukos Corporation.
Photrexa® Viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution) and Photrexa® (riboflavin 5’-phosphate ophthalmic solution) are indicated for use with the KXL System in corneal collagen cross-linking for the treatment of progressive keratoconus and corneal ectasia following refractive surgery.
1. Photrexa Prescribing Information. Burlington, MA: Avedro, a Glaukos company 2022