Solutions for Healthcare Providers
iStent inject® W
iStent inject® W is indicated for use in conjunction with cataract surgery for the reduction of IOP in adult patients with mild to moderate primary open-angle glaucoma.

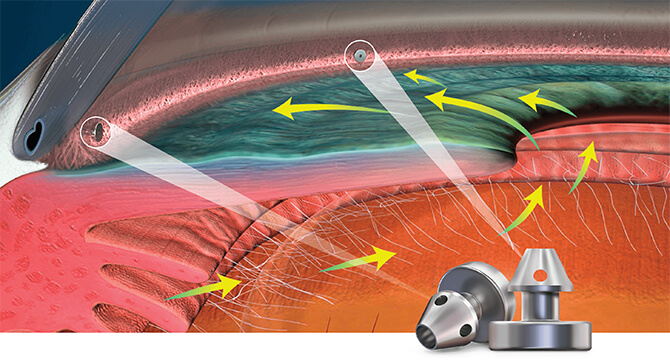
Mechanism of Action: Restoring the Natural Outflow Pathway

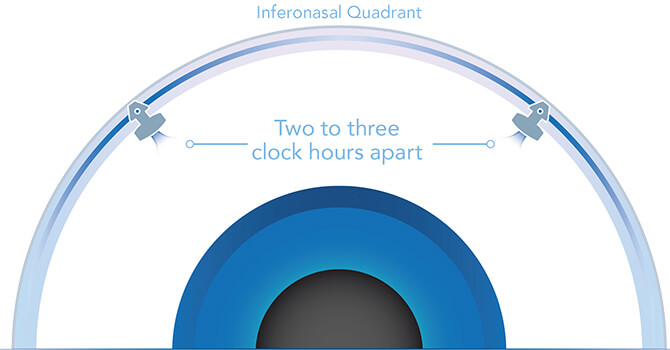
Delivering two preloaded trabecular micro-bypass stents with a single entry, iStent inject® W is designed to optimise the benefits of MIGS. The iStent inject® W device is designed to improve aqueous outflow through the natural physiological outflow pathway. When the device is implanted in the trabecular meshwork, it is designed to stent open that section of the ocular anatomy to allow aqueous humor to flow from the anterior chamber into Schlemm’s canal. A natural episcleral back pressure of 8-11 mm Hg in the trabecular meshwork reduces the risk of hypotony.4

Watch How It Works


iStent inject® W System
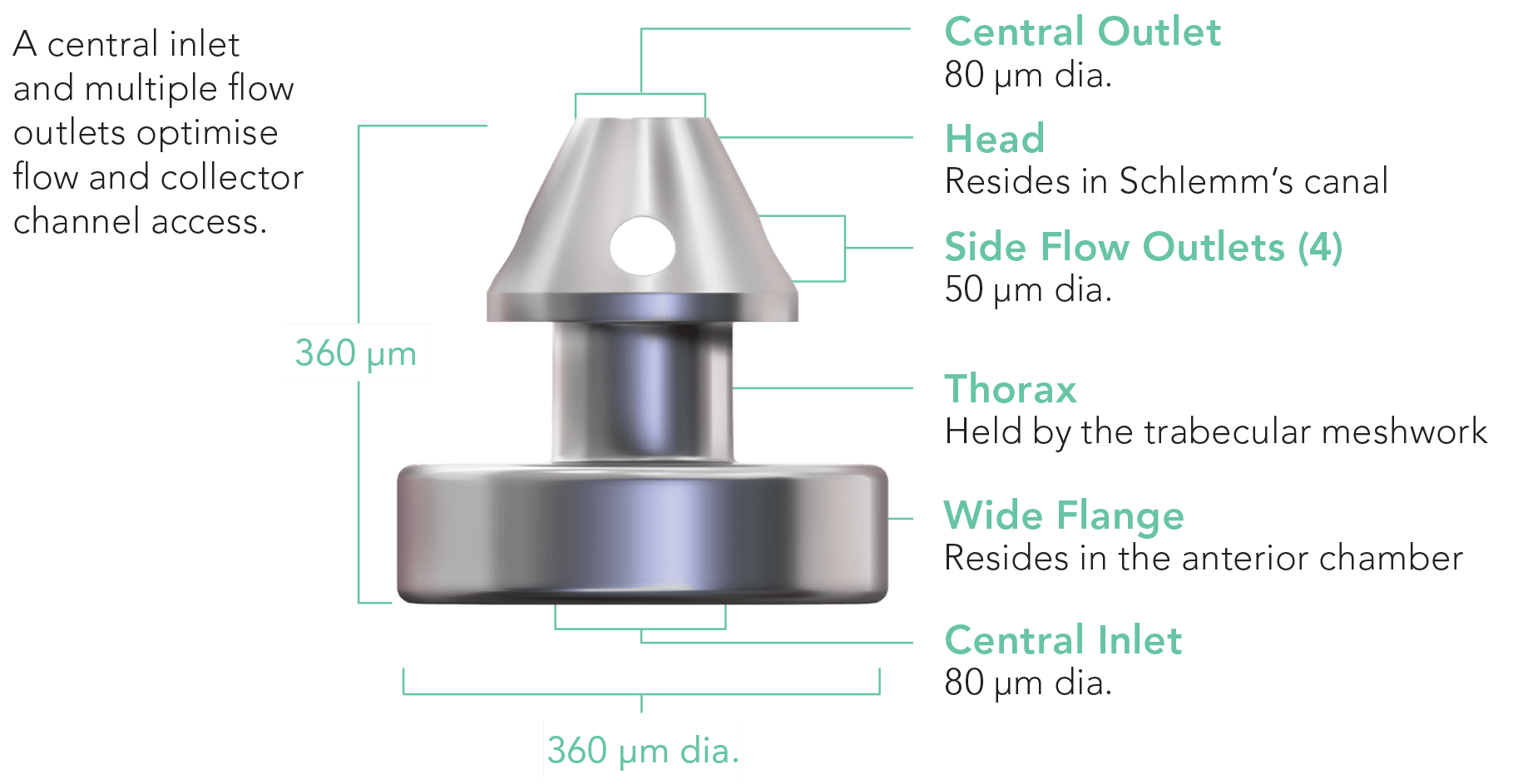
The iStent inject® W System is engineered to provide an enhanced surgical experience and ensure confident delivery, for every procedure. With a streamlined injector system and next-generation stent design, featuring a wide flange at its base, iStent inject® W is designed to optimise stent visualisation and placement, enhance procedural predictability, and increase peace of mind.

Focused on Excellent Outcomes
See the latest clinical data around iStent inject® W patient outcomes.
We’re here to answer questions and provide any additional information you may need.
Request More Info
"*" indicates required fields
References
- Lindstrom R, Lewis R, Hornbeak H, Voskanyan L, Giamporcaro JE, Hovanesian J, Sarkisian S. Outcomes Following Implantation of Two Second-Generation Trabecular Micro-Bypass Stents in Patients with Open-Angle Glaucoma on One Medication: 18-Month Follow-Up. Adv Ther 2016;33:2082-2090.
- Berdahl J, Voskanyan L, Myers JS, Hornbeak DM, Giamporcaro JE, Katz LJ, Samuelson TW. Implantation of two second-generation trabecular micro-bypass stents and topical travoprost in open-angle glaucoma not controlled on two preoperative medications: 18-month follow-up. Clin Exp Ophthalmol 2017 Nov;45(8):797-802.
- Voskanyan L, García-Feijoó J, Belda J, Fea A, Jünemann A, Baudouin C. “Prospective, Unmasked Evaluation of the iStent inject System for Open-Angle Glaucoma: Synergy Trial”. Adv Ther 2014; 31:189-201.
- Rosenquist R, Epstein D, Melamed S, Johnson M, Grant WM. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculectomy. Curr Eye Res. 1989;8:1233-1240.
