Solutions for Healthcare Providers
iStent infinite®
The iStent infinite® Trabecular Micro-Bypass System is intended to reduce intraocular pressure safely and effectively in adult patients diagnosed with primary open-angle glaucoma, pseudo-exfoliative glaucoma or pigmentary glaucoma. It is a bleb-free procedure and can be performed as standalone or combined with cataract surgery.
iStent infinite® is the only truly micro-invasive and tissue sparing treatment for moderate to advanced glaucoma patients, designed to work continuously, non-reliant on patient compliance and adherence.
iStent infinite® is based on the iStent® technologies with the most robust, diverse, and longest-term data of any Trabecular Micro- Bypass procedure today.


The Beginning of the Interventional Glaucoma Revolution
With 3 stents preloaded into a next generation injector system for delivery with unlimited opportunities, iStent infinite® is designed to:
- Offer safe interventional glaucoma for the treatment of moderate to advanced glaucoma patients helping address rampant rates of patient non-compliance and disease progression.
- Provide powerful technology to help provide IOP reductions in moderate to advanced glaucoma patients with a high preoperative burden1.
- Deliver predictable procedures with maximum precision in stent implantation through the new generation injector system
- Restore physiologic outflow by creating arcs of flow spanning up to 8 clock hours (240°) while minimizing tissue disruption-broad coverage vs other MIGS procedures2.
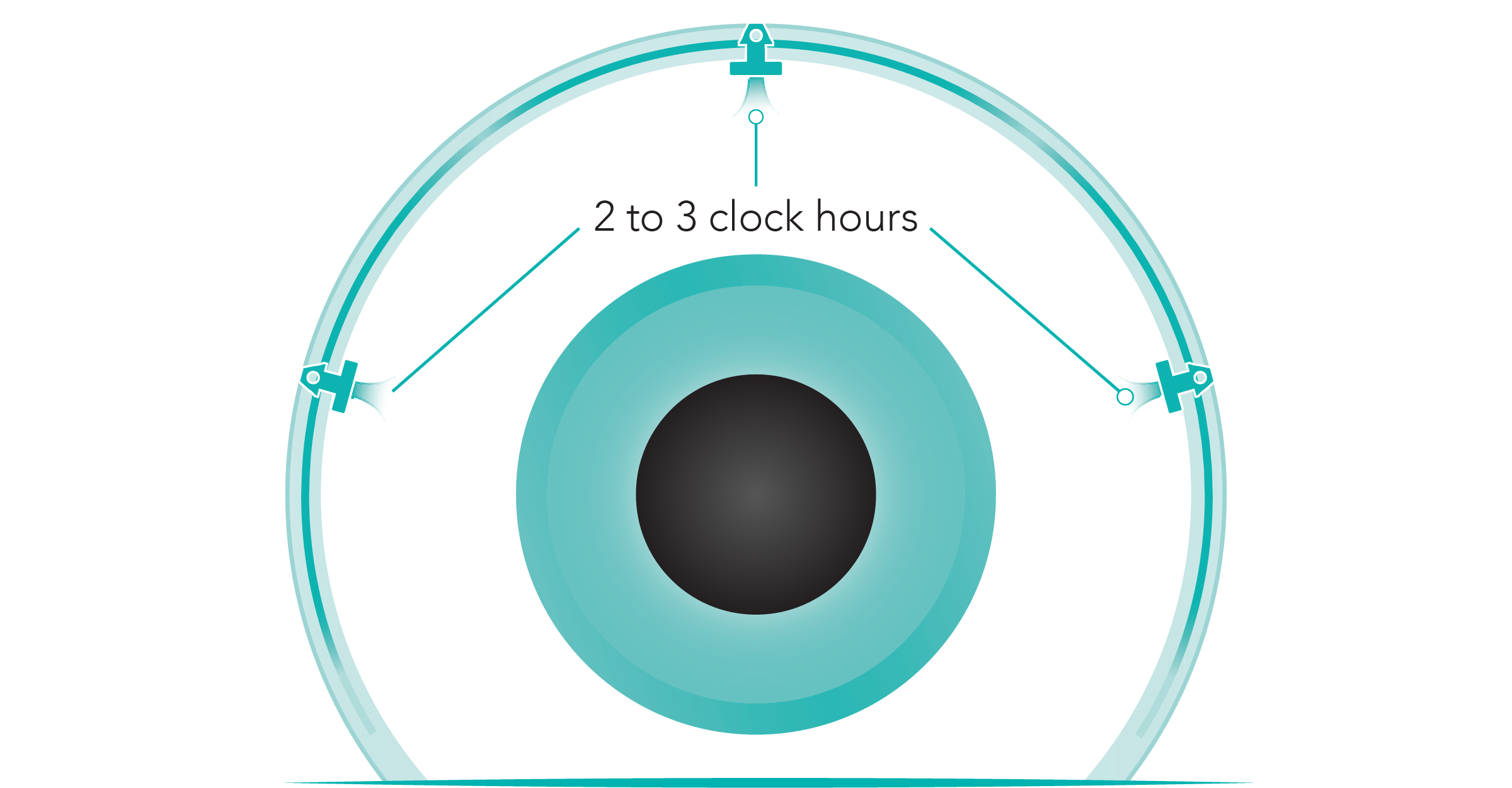
Illustration is not representative of actual anatomical structures.
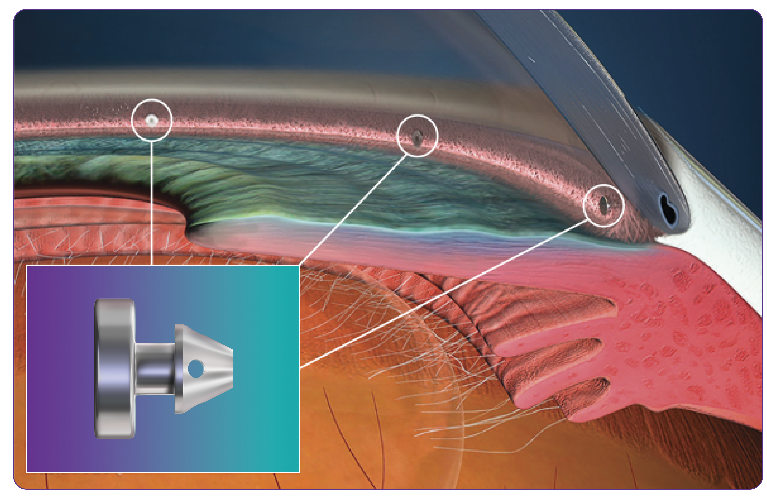
Go With the Flow—Rejuvenate the System

Illustration is not representative of actual anatomical structures.
iStent infinite® is designed to maximize outflow while minimizing disruption to natural anatomy by occupying only 3% of Schlemm’s canal, thereby leaving 97% untouched.
This, coupled with a patented multidirectional stent design, helps bypass resistance and restore physiologic outflow.

Illustration is not representative of actual anatomical structures.
Intermediate Therapy That Excels Where Other Treatments Have Failed

In the prospective, multicenter, 12-month pivotal trial, patients with a significantly high preoperative treatment burden with more severe glaucoma as compared to other trabecular bypass MIGS pivotal trials1,3,4 underwent standalone iStent infinite® implantation1.
Despite this tough-to-treat population, iStent infinite® delivered exceptional results demonstrating sustained efficacy throughout the course of the study1.
View Clinical Data
Next Generation Injector System for Optimised Delivery with Unlimited Opportunities
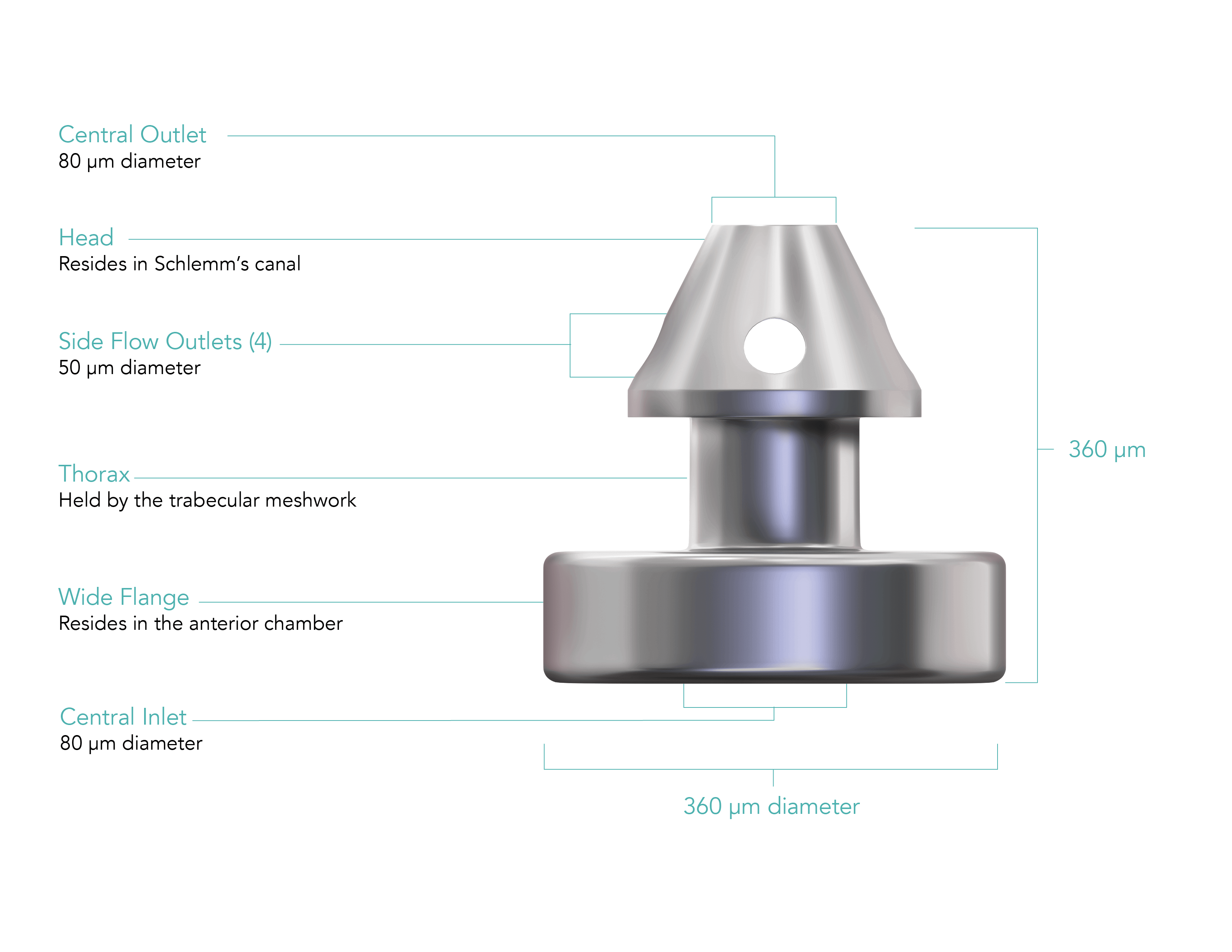
An elegant, precision-engineered injector system allows you to implant 3 wide-flange, anatomically designed stents across 180° of Schlemm’s canal.
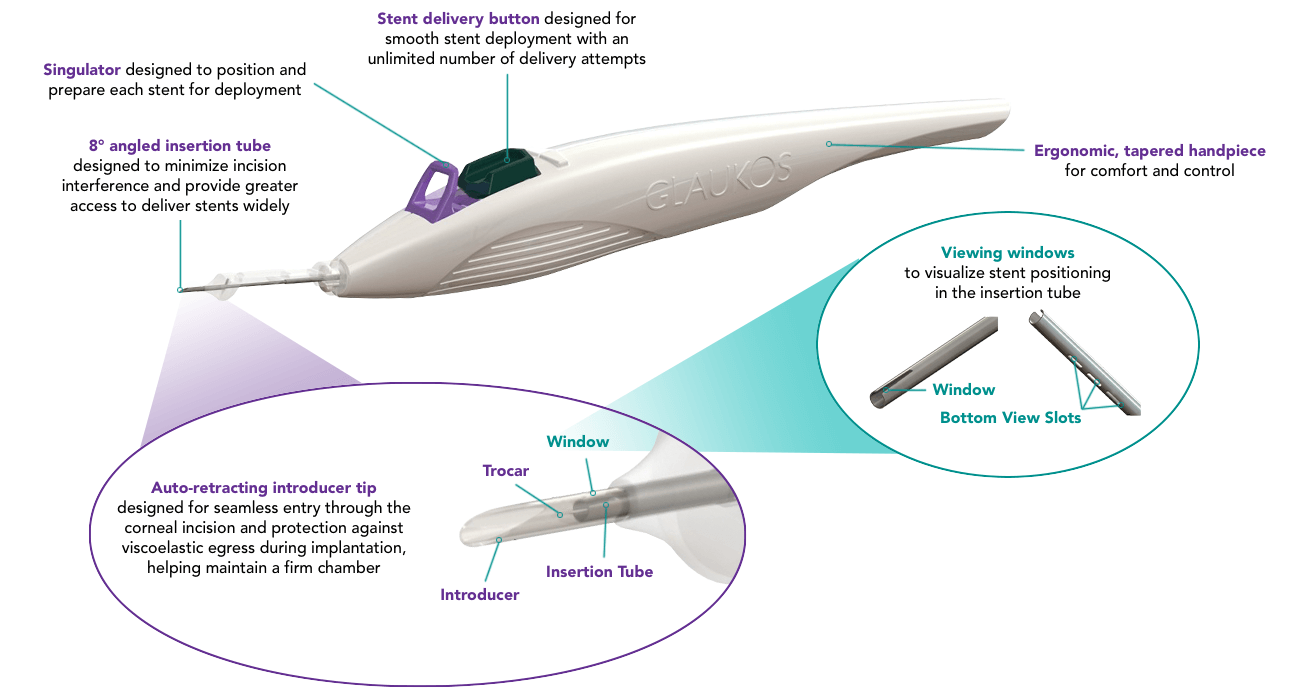
Contact Us Today to Integrate iStent infinite® Into Your Practice
Complete the form to get started with offering iStent infinite® to your patients.
Request More Info
"*" indicates required fields
- Sarkisian Jr, Steven R., et al. “Effectiveness and safety of iStent infinite trabecular micro-bypass for uncontrolled glaucoma.” Journal of glaucoma 32.1 (2023): 9-18.
- Glaukos Data on File
- CyPass. Summary of safety and effectiveness data. Alcon Laboratories, Inc; 2016.
- Samuelson TW, Chang DF, Marquis R, et al. A Schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract. Ophthalmology. 2019;126(1):29-37.
