iStent inject® W Important Safety Information
Indication For Use
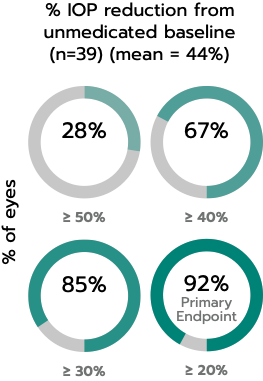
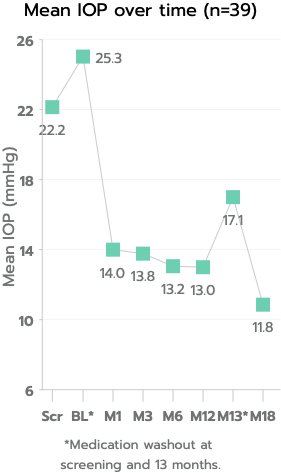
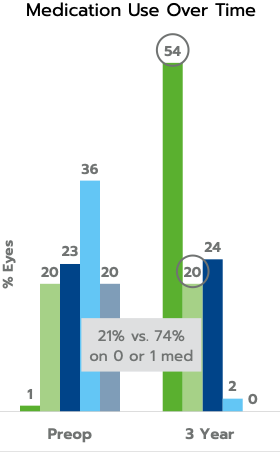
The iStent inject® W (Model G2-W) is intended to reduce intraocular pressure safely and effectively in patients diagnosed with primary open-angle glaucoma, pseudo-exfoliative glaucoma or pigmentary glaucoma. The iStent inject® W can deliver two (2) stents on a single pass, through a single incision. The implant is designed to open a passage through the trabecular meshwork allowing for increased facility of outflow and a subsequent reduction in intraocular pressure. The device is safe and effective when implanted in combination with cataract surgery in those subjects who require intraocular pressure reduction and/or would benefit from glaucoma medication reduction. The device may also be implanted in patients who continue to have elevated intraocular pressure despite prior treatment with glaucoma medications and conventional glaucoma surgery.
Contraindications
In eyes with primary angle-closure glaucoma, or secondary angle-closure glaucoma, including neovascular glaucomas, in patients with retrobulbar tumor, thyroid eye disease, Sturge-Weber Syndrome or any other type of condition that may cause elevated episcleral venous pressure.
Warnings
This device has not been studied in patients with uveitic glaucoma. The surgeon should monitor the patient postoperatively for proper maintenance of intraocular pressure. iStent inject® W is MR-Conditional meaning that the device is safe for use in a specified MRI environment under specified conditions; please see labeling for details. Physician training is required prior to use. Do not re-use the stent(s) or inserter.
Adverse Events
Postoperative adverse events include but are not limited to: early postoperative corneal edema, posterior capsule opacification, stent obstruction, intraocular inflammation (non-preexisting), BCVA loss, and IOP increase requiring management with oral or intravenous medications or surgical intervention. Please refer to Directions for Use for additional adverse event information.
Caution
Please reference Directions for Use labeling for a complete list of contraindications, warnings and adverse events