Lasik Xtra®
Enhancing LASIK
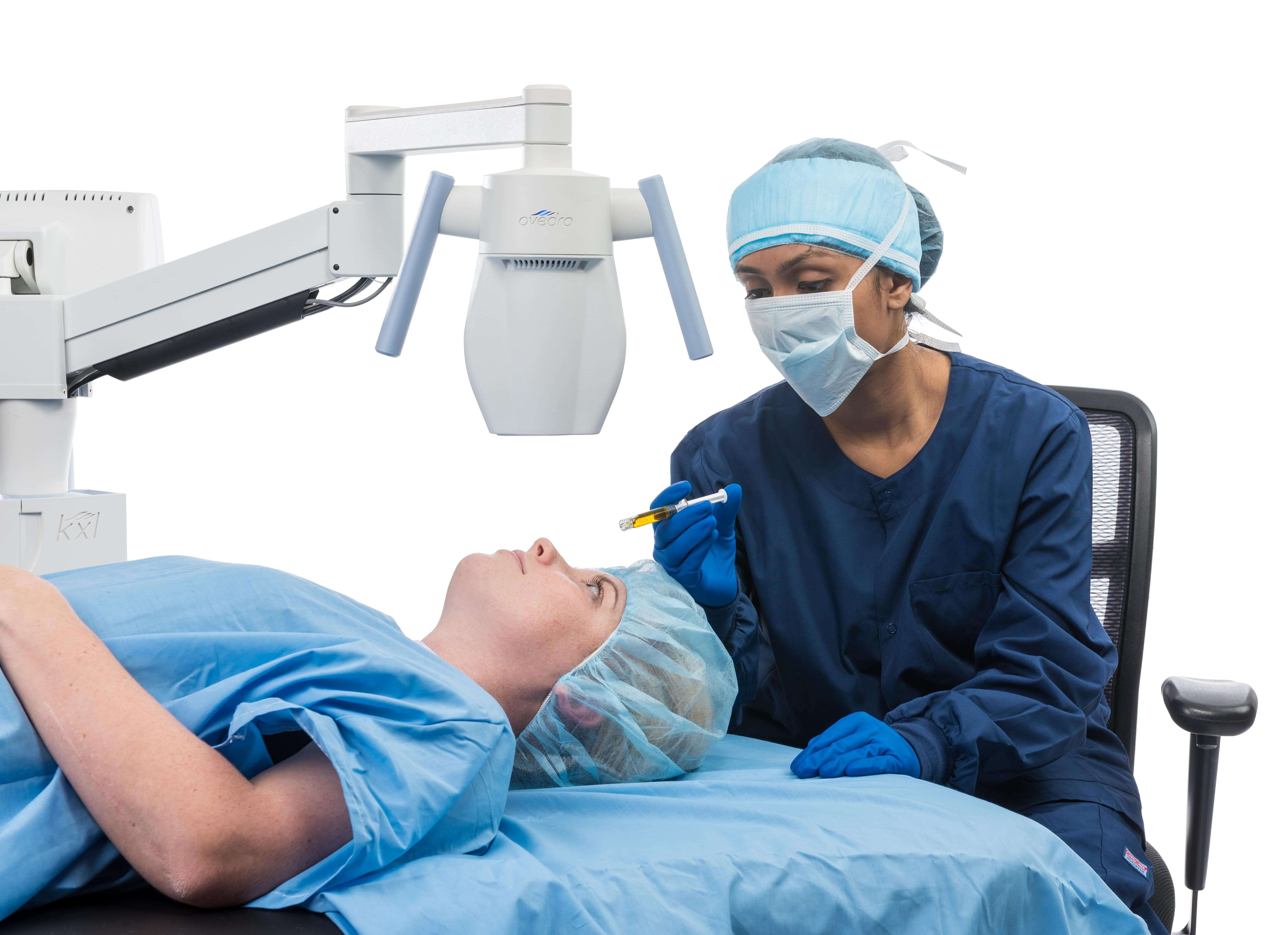
Performed in conjunction with LASIK, the Lasik Xtra procedure enhances the corneal biomechanical integrity. Lasik Xtra poses no material interruption to the normal speed and flow of the LASIK procedure and does not increase patient discomfort.
Who can benefit from Lasik Xtra?
LASIK patients:
When the flap is created during a standard LASIK procedure, the structural integrity of the cornea can be weakened by up to 33% depending on the thickness of the flap.1 Lasik Xtra has the ability to strengthen a cornea that may have been compromised during LASIK, restoring the biomechanical integrity of the eye.
How it works
After the refractive correction has been made in a standard LASIK procedure, a riboflavin formulation is applied to the exposed stroma and the flap is replaced. UVA illumination from the KXL® system is then applied through the intact epithelium for approximately 90 seconds.
Combining Corneal Cross-Linking with LASIK

By Lim Li, MD, The Ophthalmologist (September 2019)
References
- Jaycock PD, Lobo L, Ibrahim J, Tyrer J, Marshall J. Interferometric technique to measure biomechanical changes in the cornea induced by refractive surgery. J Cataract Refract Surg. 2005;31(1):175-184.
We want to hear from you!
Please contact us to learn more about our cross-linking products and procedures.
Contact Us
"*" indicates required fields
