Solutions for Healthcare Providers
iStent infinite®
Can be Used in Patients Undergoing Cataract Surgery or a Standalone Procedure
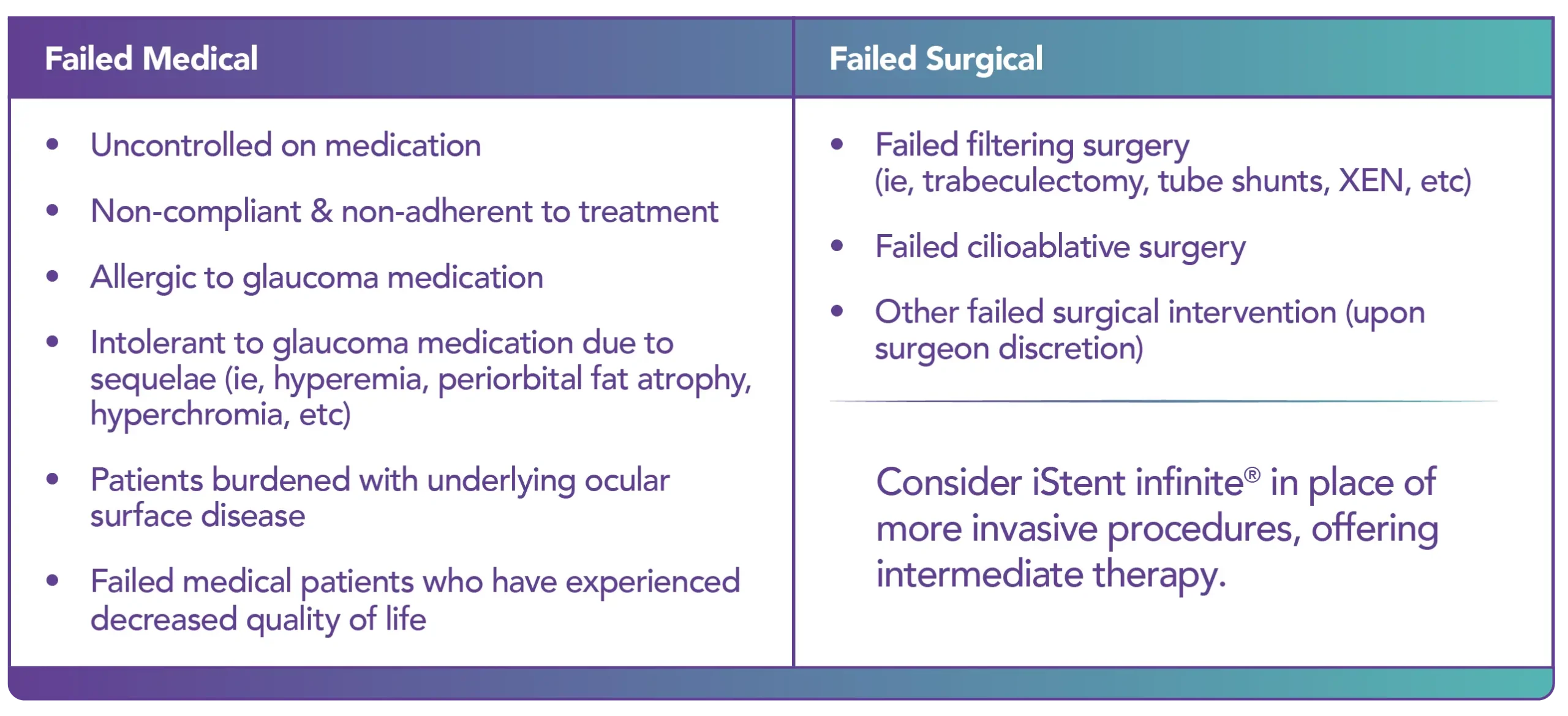
The iStent infinite® Trabecular Micro-Bypass System Model iS3 is an implantable device intended to reduce the intraocular pressure (IOP) of the eye. It is indicated for use in adult patients with primary open-angle glaucoma in whom previous medical and surgical treatment has failed.
iStent infinite® is the first micro-invasive standalone implantable alternative.


Can be Used in Patients Undergoing Cataract Surgery or a Standalone Procedure
iStent infinite® gives you the versatility to treat a variety of patients who have failed prior medical and surgical intervention, when combined with cataract surgery or in a standalone surgical setting.
The Beginning of the Interventional Glaucoma Revolution
With 3 anatomically designed stents preloaded into an elegant injector system, iStent infinite® is a first-of-its-kind, standalone implantable alternative designed to:
- Provide powerful technology to deliver foundational, 24/7, long-term control of IOP in patients with glaucoma who have failed prior medical and surgical intervention1
- Safely offer interventional glaucoma, a truly micro-invasive alternative to medications and more invasive procedures, helping address rampant rates of patient non-compliance and disease progression
- Restore physiologic outflow by creating arcs of flow spanning up to 8 clock hours (240°) while minimizing tissue disruption—broad coverage vs other MIGS procedures1
- Provide you the versatility to treat a variety of patients
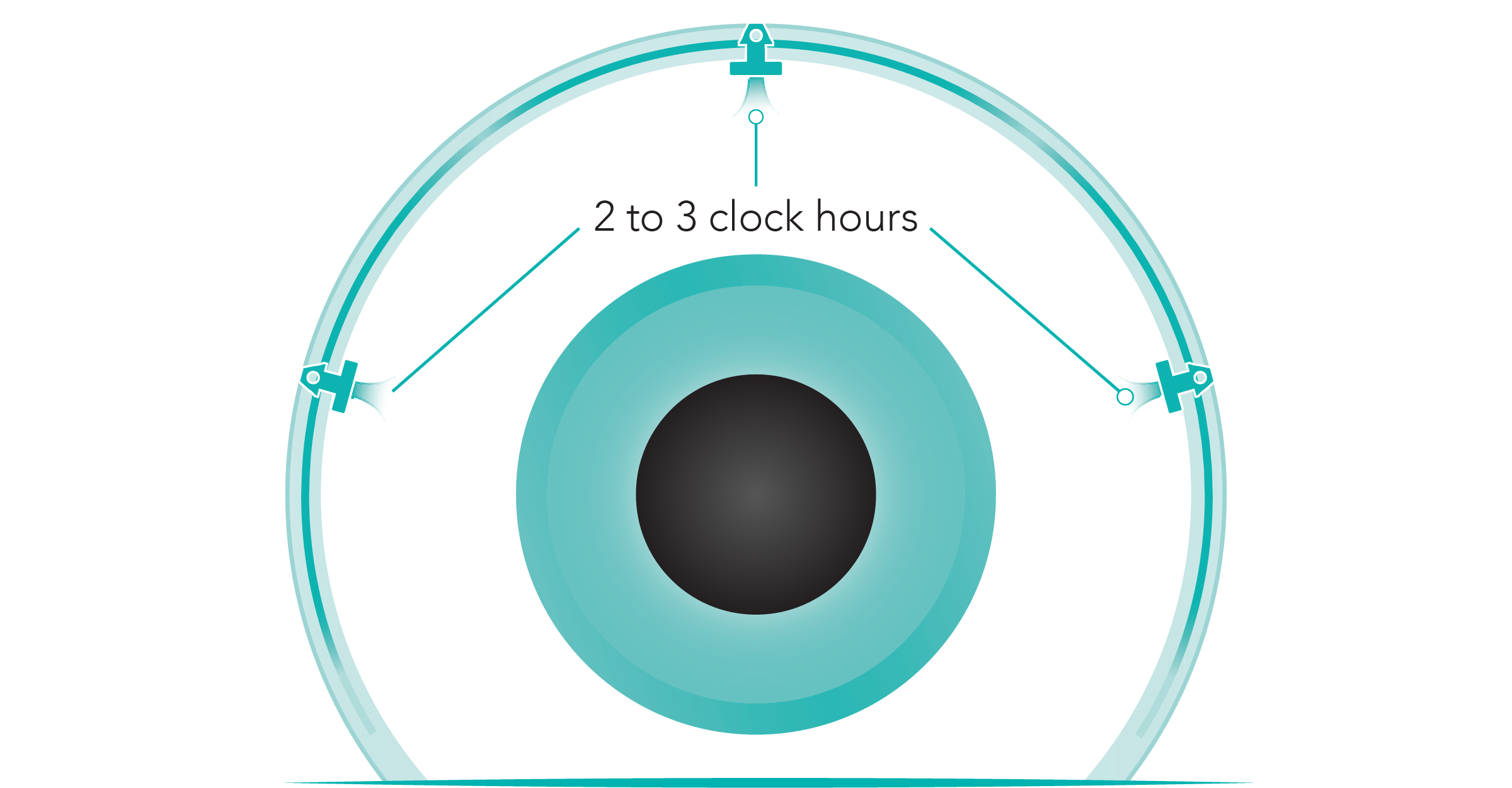
Illustration is not representative of actual anatomical structures.
Go With the Flow—Rejuvenate the System

Illustration is not representative of actual anatomical structures.
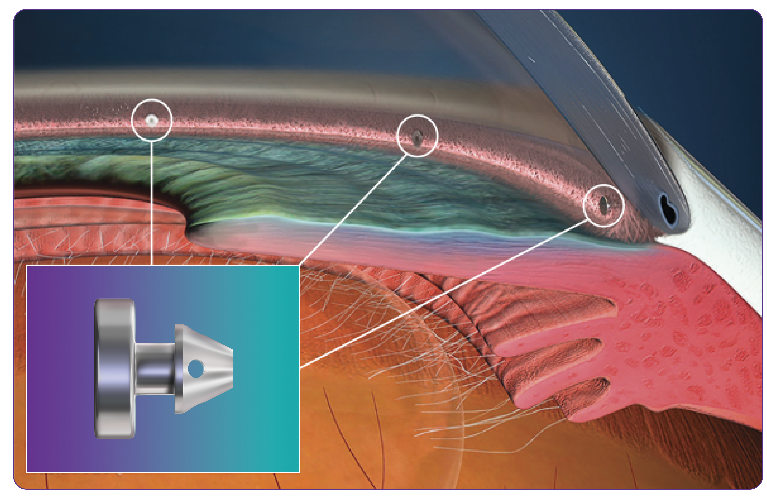
iStent infinite® is designed to maximize outflow while minimizing disruption to natural anatomy by occupying only 3% of Schlemm’s canal, thereby leaving 97% untouched.
This, coupled with a patented multidirectional stent design, helps bypass resistance and restore physiologic outflow.

Illustration is not representative of actual anatomical structures.
Intermediate Therapy That Excels Where Other Treatments Have Failed

In the prospective, multicenter, 12-month pivotal trial, patients with open-angle glaucoma who had failed prior surgical intervention underwent standalone iStent infinite® implantation1.
Despite this tough-to-treat population, iStent infinite® delivered exceptional results demonstrating sustained efficacy throughout the course of the study, as well as exceptional intraoperative and postoperative safety1.
View Clinical Data
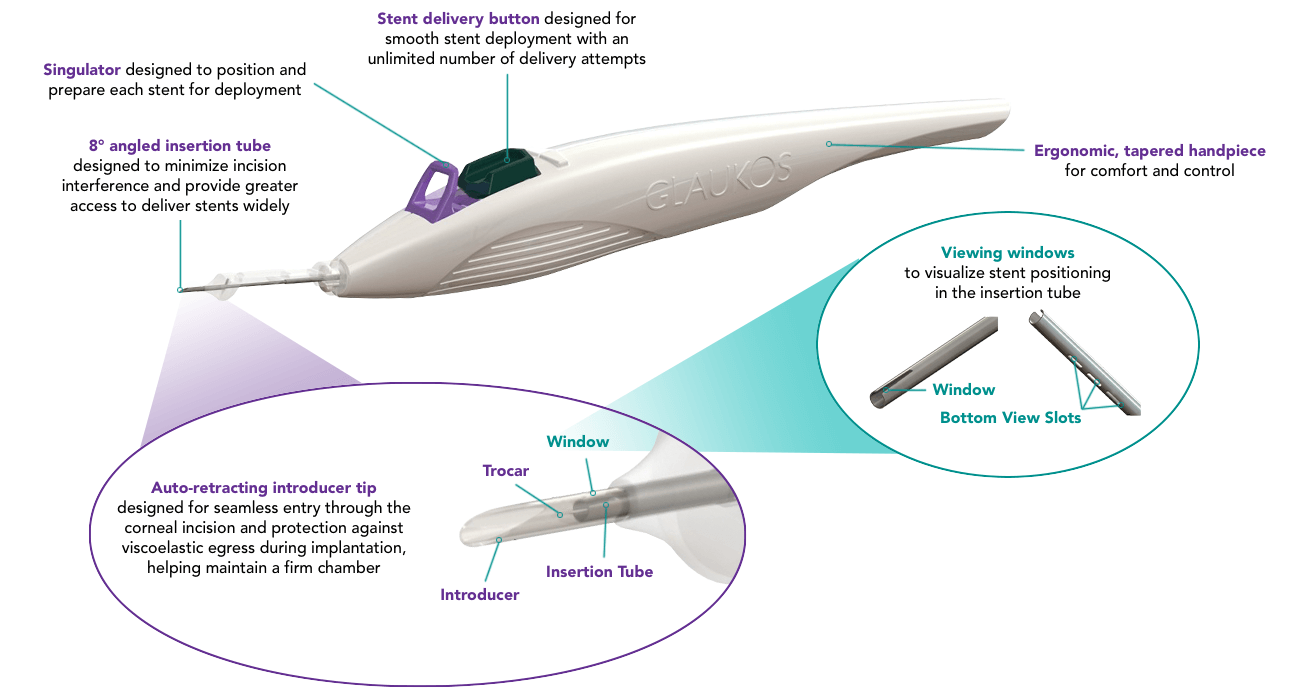
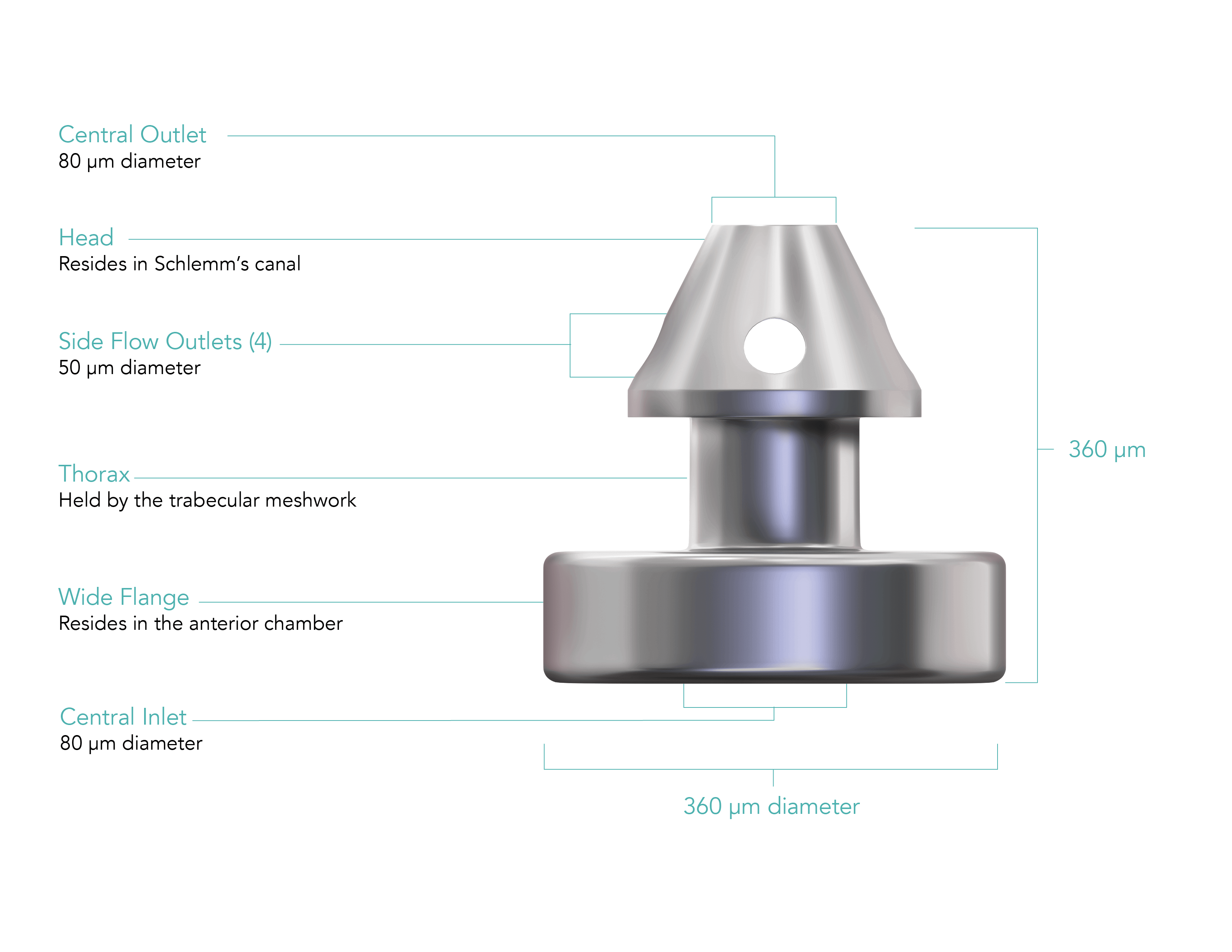
Elegantly Precise Microsurgery
An elegant, precision-engineered injector system allows you to implant 3 wide-flange, anatomically designed stents across 180° of Schlemm’s canal.
Explore Patient Resources for Glaucoma Treatment Options
iStent infinite® is indicated for use in adult patients with primary open-angle glaucoma in whom previous medical and surgical treatment has failed. Through this procedure, you may be able to provide your patients with better IOP control and a reduced dependence on medication.
Reimbursement Support
Tailor-Made Market Access Solutions
Looking for reimbursement info? GPS (Glaukos Patient Services) provides strategic and trustworthy market access solutions for all Glaukos procedures and products in glaucoma, corneal health, and retinal disease care.
Resources for Healthcare Professionals
Resources to Help You Make Informed Decisions
As a trusted industry leader – and the corporate founder of MIGS – we have the experience, tools, and training to make integration easy, starting with the resources we make available to you.
Contact Us Today to Integrate iStent infinite® Into Your Practice
Complete the form to get started with offering iStent infinite® to your patients.
Request More Info
"*" indicates required fields
Reference
- Glaukos Data on File.
