Solutions for Healthcare Providers
iStent inject® W
iStent inject® W is indicated for use in conjunction with cataract surgery for the reduction of IOP in adult patients with mild to moderate primary open-angle glaucoma.
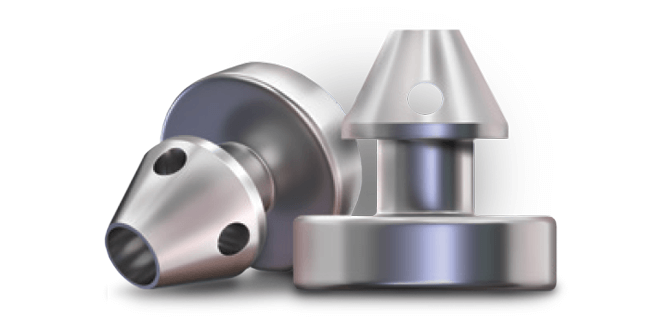

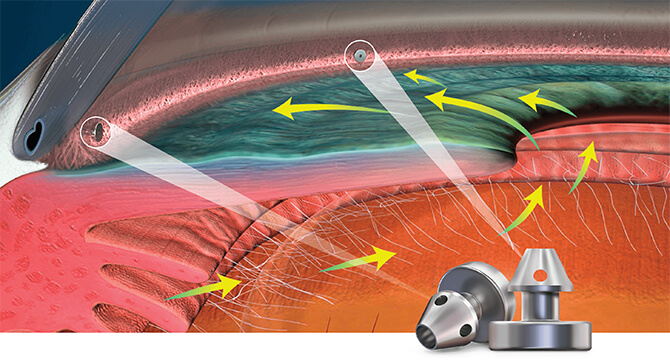
Mechanism of Action: Restoring the Natural Outflow Pathway

iStent inject® W creates two patent bypass pathways through the trabecular meshwork—the main source of resistance for aqueous outflow—resulting in multi-directional flow through Schlemm’s canal. And it is one of the smallest medical devices known to be implanted in the human body. Together, these unique advantages are designed to provide exceptional results in a truly micro-invasive approach.

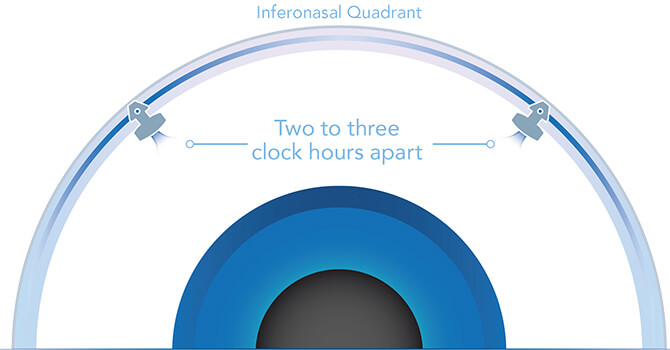
Optimize Outflow with the iStent inject® W

Glaukos trabecular micro-bypass stents are placed two to three clock hours apart. The stents are multi-directional and together, they are designed to deliver access to multiple collector channels and arcs of flow that can span five to six clock hours. The stents may also re-establish flow in previously dormant outflow channels.3

Prioritizing Safety and Outcomes
Putting the primary emphasis on maximizing benefits and minimizing long-term complications, iStent inject® W is designed to support optimal outcomes after cataract surgery and much more:
- Micro-invasive and astigmatically-neutral
- Utilizes the conventional outflow pathway
- Leaves natural anatomy intact, preserving the potential for future treatment options, including drug delivery devices
- Minimally traumatic to delicate eye tissue and spares conjunctival tissue
- Reduces risk of hypotony by utilizing the natural episcleral venous pressure
- Offers postoperative care profile similar to cataract surgery
Exceptional Elegance. Advanced Innovation
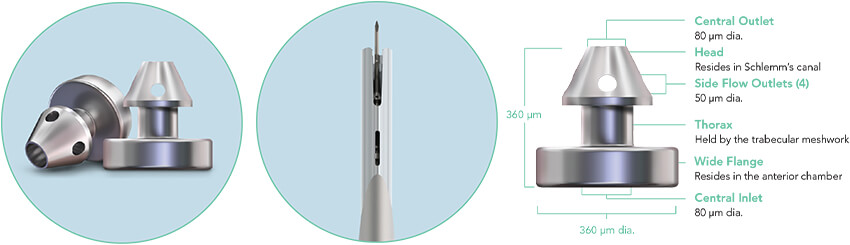
The iStent inject® W System is engineered to provide an enhanced surgical experience and ensure confident delivery, for every procedure. With a streamlined injector system and next-generation stent design, featuring a wide flange at its base, iStent inject® W is designed to optimize stent visualization and placement, enhance procedural predictability, and increase peace of mind.

Explore Patient Resources for Glaucoma Treatment Options
iStent inject® W is indicated for use in adult patients with mild-to-moderate open-angle glaucoma that are undergoing cataract surgery. By taking advantage of this one-time opportunity, you may be able to provide your patients with better IOP control and a reduced dependence on medication.
Micro Stents. Macro Efficacy.
See the latest clinical data around patient outcomes. iStent inject® W builds on the trabecular micro-bypass technology of iStent inject®, which has demonstrated efficacy across a wide range of clinical studies.
Reimbursement Support
Tailor-Made Market Access Solutions
Looking for reimbursement info? The iPath360 program provides strategic and trustworthy market access solutions for all Glaukos procedures and products in glaucoma, corneal health, and retinal disease care.
Resources for Healthcare Professionals
Resources to Help You Make Informed Decisions
As a trusted industry leader – and the corporate founder of MIGS – we have the experience, tools, and training to make integration easy, starting with the resources we make available to you.
Contact Us Today to Integrate iStent inject® W Into Your Practice
Complete the form to get started with offering iStent inject® W to your patients.
Request More Info
"*" indicates required fields
References
- Samuelson TW, Sarkisian SR, Lubeck DM, et al. Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract. Ophthalmology. Jun 2019;126(6):811-821.
- Hengerer FH, Auffarth GU, Riffel C, Conrad-Hengerer I. Prospective, non-randomized, 36-month study of second-generation trabecular micro-bypass stents with phacoemulsification in eyes with various types of glaucoma. Ophthalmol Ther. 2018 Dec;7(2):405-415.
- Data on file, Glaukos Corporation
