The iLink® Procedure
iLink® Corneal Cross-Linking
iLink® corneal cross-linking is an innovative therapy that has transformed the treatment of progressive keratoconus. This minimally invasive outpatient procedure uses Photrexa® Viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution) and Photrexa® (riboflavin 5’-phosphate ophthalmic solution) riboflavin eye drops, combined with ultraviolet light from the KXL system to1:
- Create new corneal collagen cross-links
- Shorten and thicken collagen fibrils
- Stiffen and strengthen the cornea
Solutions For Healthcare Providers
Solutions For Healthcare Providers
Transforming the Standard of Care for Progressive Keratoconus
iLink® is the first and only FDA-approved corneal cross-linking procedure that slows or halts the progression of keratoconus and helps preserve vision.
Watch this video for unique patient and doctor perspectives about progressive keratoconus and learn how this underserved patient population with a rare and sight-threatening condition is treated with iLink® corneal cross-linking.
Contact RepresentativeThe Proprietary iLink® Corneal Cross-Linking Procedure

Left untreated, 1 in 5 patients with progressive keratoconus may require a corneal transplant. As this disease is typically diagnosed in teens or early twenties, more than half of these patients could need multiple transplants within 20 years. 2,3
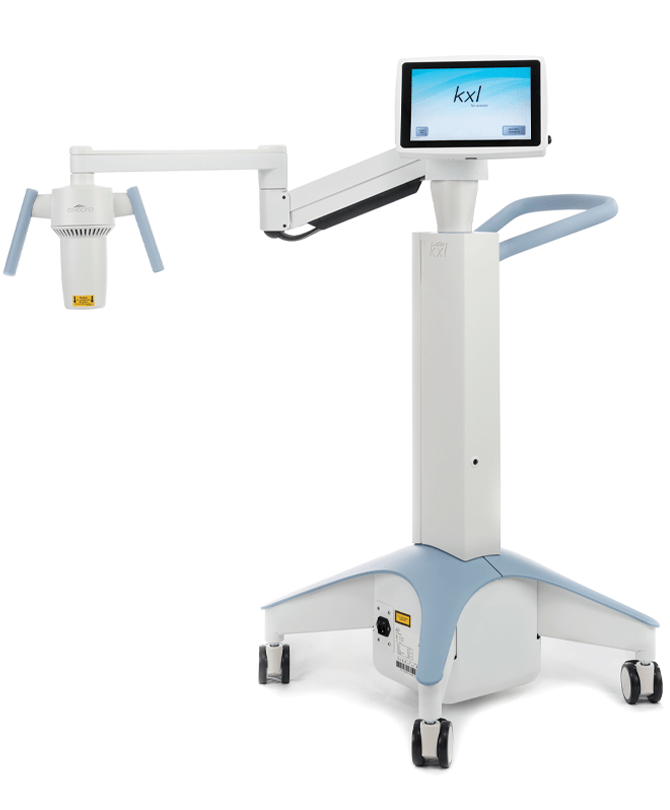
The KXL System
- UVA irradiation: 30 minutes at 3 mW/cm²
- Laser alignment for patient positioning
- Wireless control for beam alignment in the X, Y, and Z axes
- Fully integrated stable delivery platform
- Touchscreen operation
- Self-calibration of UVA irradiation intensity
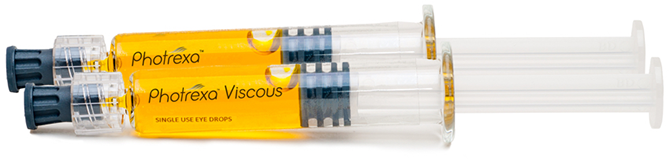
Photrexa® Viscous (riboflavin 5’-phosphate in 20% dextran ophthalmic solution) and Photrexa® (riboflavin 5’-phosphate ophthalmic solution)
- Only FDA-approved bioactivated riboflavin ophthalmic solutions
- Manufactured using validated processes in a state-of-the-art drug manufacturing facility
- GMP-certified material
- Packaged and controlled under GMP standards
 Download System Operator Manuals
Download System Operator Manuals
Explore The Results of Our Clinical Study Data
In clinical studies, cross-linking–treated eyes showed increasing improvement in Kmax from month 3 through month 12, while in untreated, sham eyes, Kmax demonstrated steepening.4
If it’s not iLink®, it’s not FDA approved
When it comes to referring patients with progressive keratoconus for treatment, iLink® is the only FDA-approved corneal cross-linking procedure.
Explore Patient Resources for Keratoconus
Treatment Options
View Patient Website
Reimbursement Support
Tailor-Made
Market
Access Solutions
Looking for reimbursement info? GPS (Glaukos Patient Services) provides strategic and trustworthy market access solutions for all Glaukos procedures and products in glaucoma, corneal health, and retinal disease care.
Resources for Healthcare Professionals
Learn More About iLink® from the Experts
Order print versions of our practice marketing materials, including educational resources on keratoconus and FDA-approved cross-linking. You can also download digital versions of many of our educational resources.
Contact Us Today
to Integrate the iLink®
Procedure Into Your
Practice
Explore additional information about iLink® in our digital brochure and learn how the procedure is transforming the standard of care for progressive keratoconus.
Request
More Info
"*" indicates required fields
References
- Beshtawi IM, O’Donnell C, Radhakrishnan H. Biomechanical properties of corneal tissue after ultraviolet-A-riboflavin crosslinking. J Cataract Refract Surg. 2013;39(3):451–462.
- Pramanik S, Musch DC, Sutphin JE, Farjo AA. Extended long-term outcomes of penetrating keratoplasty for keratoconus. Ophthalmology. 2006;113(9):1633-1638.
- Maharana PK, Agarwal K, Jhanji V, Vajpayee RB. Deep anterior lamellar keratoplasty for keratoconus: a review. Eye Contact Lens. 2014;40(6):382-389.
- Photrexa [package insert]. Waltham, MA: Glaukos, Inc. 2016.
