Riboflavin Family
CE-marked riboflavin formulations
Our scientifically developed family of CE marked riboflavin formulations—VibeX Xtra™, VibeX Rapid™, ParaCel™, MedioCROSS® TE, MedioCROSS® M, MedioCROSS® D, and MedioCROSS® H—ensure rapid diffusion in individual, sterile syringes for all your cross-linking needs.
We are proud to offer multiple CE-marked riboflavin formulations, which have set the standard to produce the highest quality riboflavin since the introduction of corneal cross-linking.
Glaukos riboflavin formulations have been used in hundreds of thousands of treatments around the world.
A tradition of quality & excellence
- Long-established riboflavin supply combining innovative science and research with German manufacturing excellence
- CE-marked products available worldwide
- Compliant with Annex V of EC-Directive 93/42/EEC
- ISO 13485 certified manufacturer and facility
- Produced in world-class clean room; sterilized using validated and monitored processes
Our riboflavin formulations have been specifically designed to address the following cross-linking protocols
Epithelium-off treatment of keratoconus and corneal ectasia
Trans-epithelial treatment of keratoconus and corneal ectasia
Intra-stromal applications in conjunction with LASIK (Lasik Xtra®)
Riboflavin
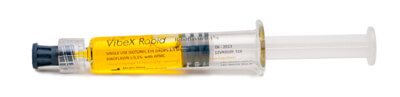
VibeX Rapid™

CE-marked VibeX Rapid™ riboflavin is formulated to improve diffusion rates while decreasing corneal thinning. VibeX Rapid has a diffusion rate of twice that of standard riboflavin and is topically applied to the cornea after epithelium removal. UVA light from the KXL® system is then applied for cross-linking.

Technical Information
|
Formulation: 0.1% Riboflavin, Saline, HPMC |
Procedure: Cross-linking for keratoconus & |
Application: Epithelium-off |
Riboflavin
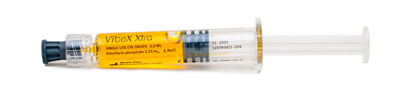
VibeX Xtra™

CE-marked VibeX Xtra riboflavin is formulated specifically for use during the Lasik Xtra® procedure. VibeX Xtra is applied directly to the stromal bed prior to replacing the flap following excimer laser ablation. Formulated in an isotonic solution, VibeX Xtra diffuses quickly to minimize flap exposure time and deliver the appropriate concentration of riboflavin for cross-linking. Once the flap has been repositioned over the cornea, UVA light from the KXL® system is applied to activate VibeX Xtra™ to restore biomechanical integrity to the cornea.

Technical Information
|
Formulation: 0.22% Riboflavin, Saline, Isotonic |
Procedure: |
Application: Stromal Bed |
Riboflavin
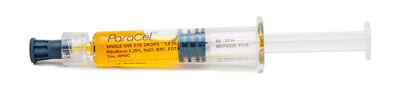
ParaCel™

CE-marked ParaCel is a 2-step trans-epithelial riboflavin specifically formulated for direct application on the intact epithelium. ParaCel’s higher concentration of riboflavin and proprietary formulation allows for fast penetration and diffusion into the corneal stroma.
Intact epithelium diminishes the effects of cross-linking when low-powered UVA devices are used. The KXL® system offers 45 mW/cm2 of power for effective cross-linking through the intact epithelium using ParaCel.

Technical Information
|
Formulation: 0.25% Riboflavin, HPMC, BAC |
Procedure: Cross-linking for keratoconus & |
Application: Epithelium-on |
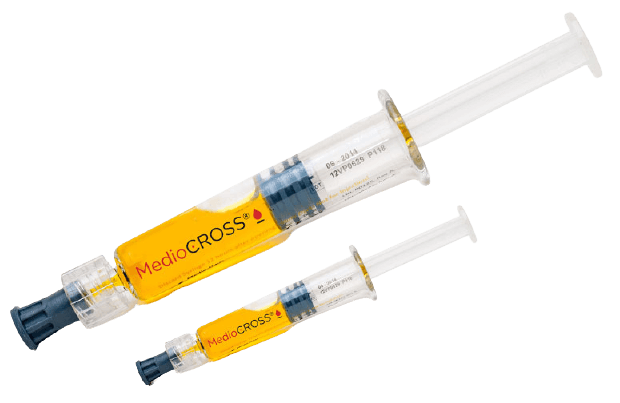
MedioCROSS®
MedioCROSS®
Glaukos is also the exclusive distributor of MedioCROSS® riboflavin. Please contact us or click below for more information on our line of MedioCROSS® riboflavin formulations.
We want to hear from you!
Please contact us to learn more about our cross-linking products and procedures.
Contact Us
"*" indicates required fields
