Solutions For Healthcare Providers
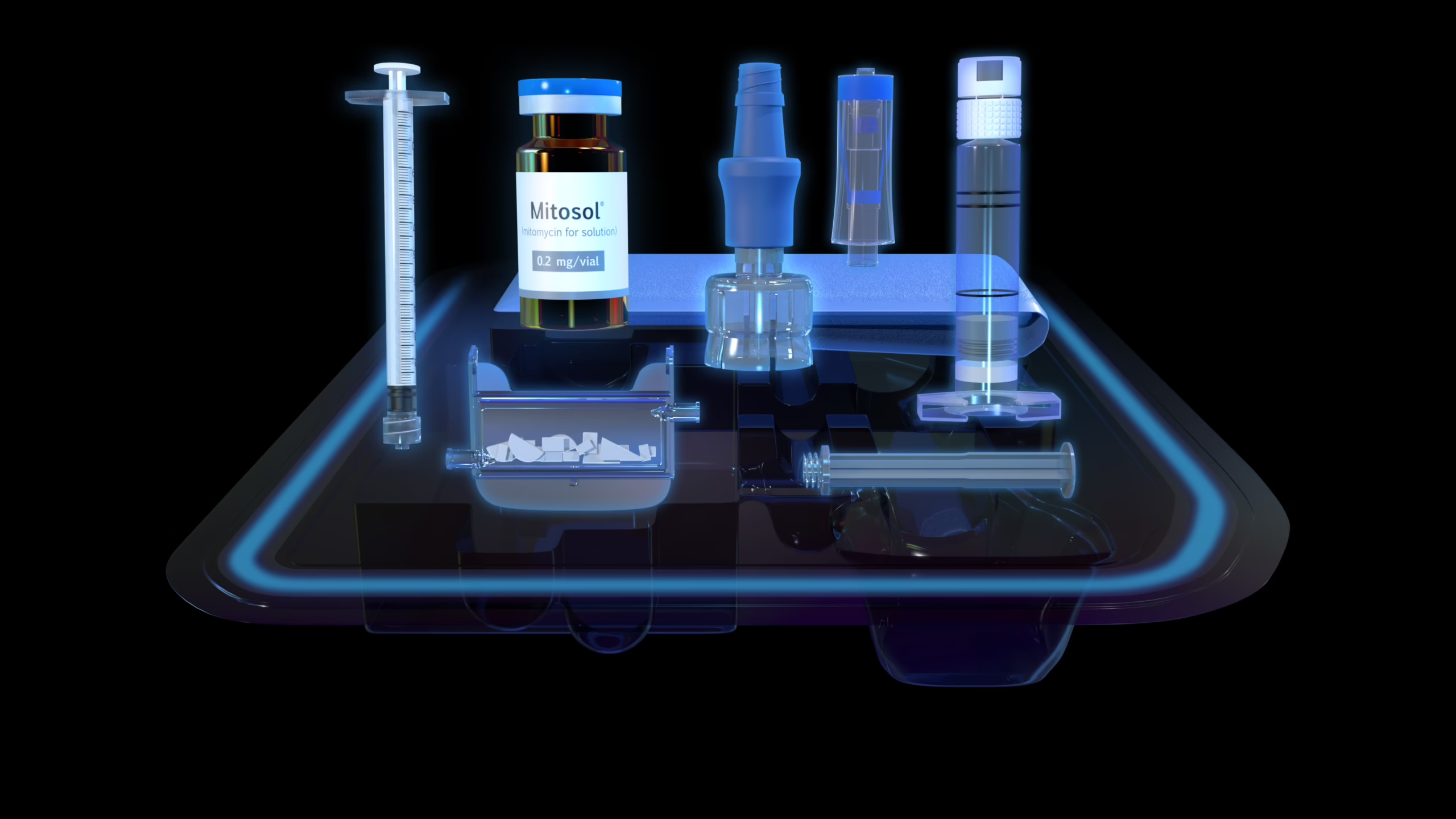
Mitosol® (mitomycin for solution) 0.2 mg/vial Kit for Ophthalmic Use
Mitosol is an antimetabolite which received FDA approval in 2012 as an adjunct to ab-externo glaucoma surgery. Mitosol is the only FDA approved ophthalmic Mitomycin C formulation.

Meet the standard of care in glaucoma surgery
-
FDA
The only formulation of ophthalmic MMC approved by the FDA
-
USP<800>
The only system to meet new USP<800> standardsfor containment and disposal
-
CSTD
Closed-system transfer device minimizes risk of breakage, leakage, and exposure
FDA-approved ophthalmic MMC
The Total Package
- Offers an on-demand reconstitution system that’s as immediate as it is precise
- Can be stored at room temperature for up to 24 months
- Patented, closed-system preparation and disposal
Resources for Healthcare Professionals
Resources to Help Make Informed Decisions
As a trusted industry leader – and the corporate founder of MIGS – we have the experience, tools, and training to make integration easy, starting with the resources we make available to you.
Contact Us Today to Integrate Mitosol® Into Your Practice
Complete the form to get started with offering Mitosol to your patients.
Request More Info
"*" indicates required fields
The information contained herein is intended for use by US healthcare professionals.
