ADVANCING THE STANDARD OF GLAUCOMA CARE
Interventional Glaucoma
For decades, glaucoma management has been centered around two treatment approaches—topical medications and incisional surgery. Discover how interventional glaucoma is reshaping the treatment landscape for patients with this sight threatening disease.1

Re-examining The Conventional Glaucoma Patient Journey
Reactive glaucoma management follows a stepwise approach, escalating treatment only after disease progression.1,2
The current “medication-first and always” mindset is being challenged
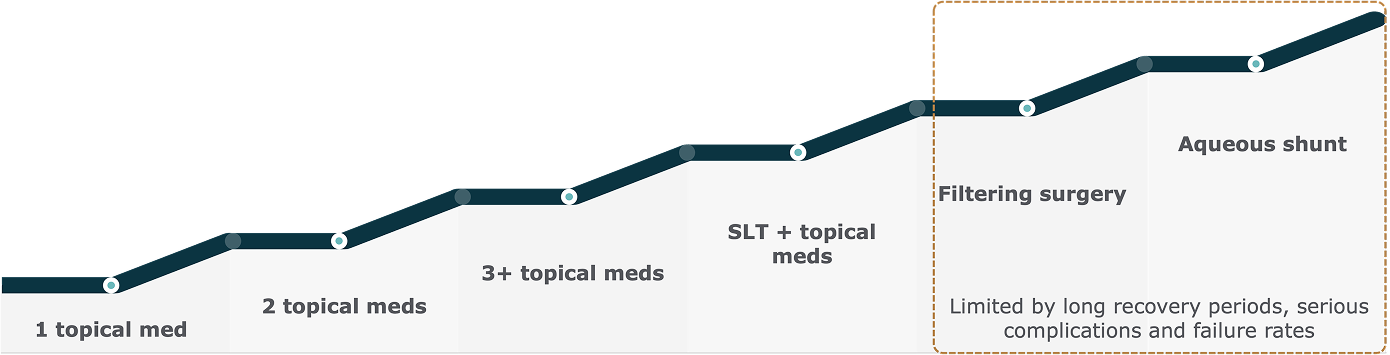
Limitations of Glaucoma Drop Therapy
Patients treated with glaucoma drop therapy regimens can suffer significant disease progression2
Probability of Primary Open Angle Glaucoma-related Blindness by Visual Field or Visual Acuity
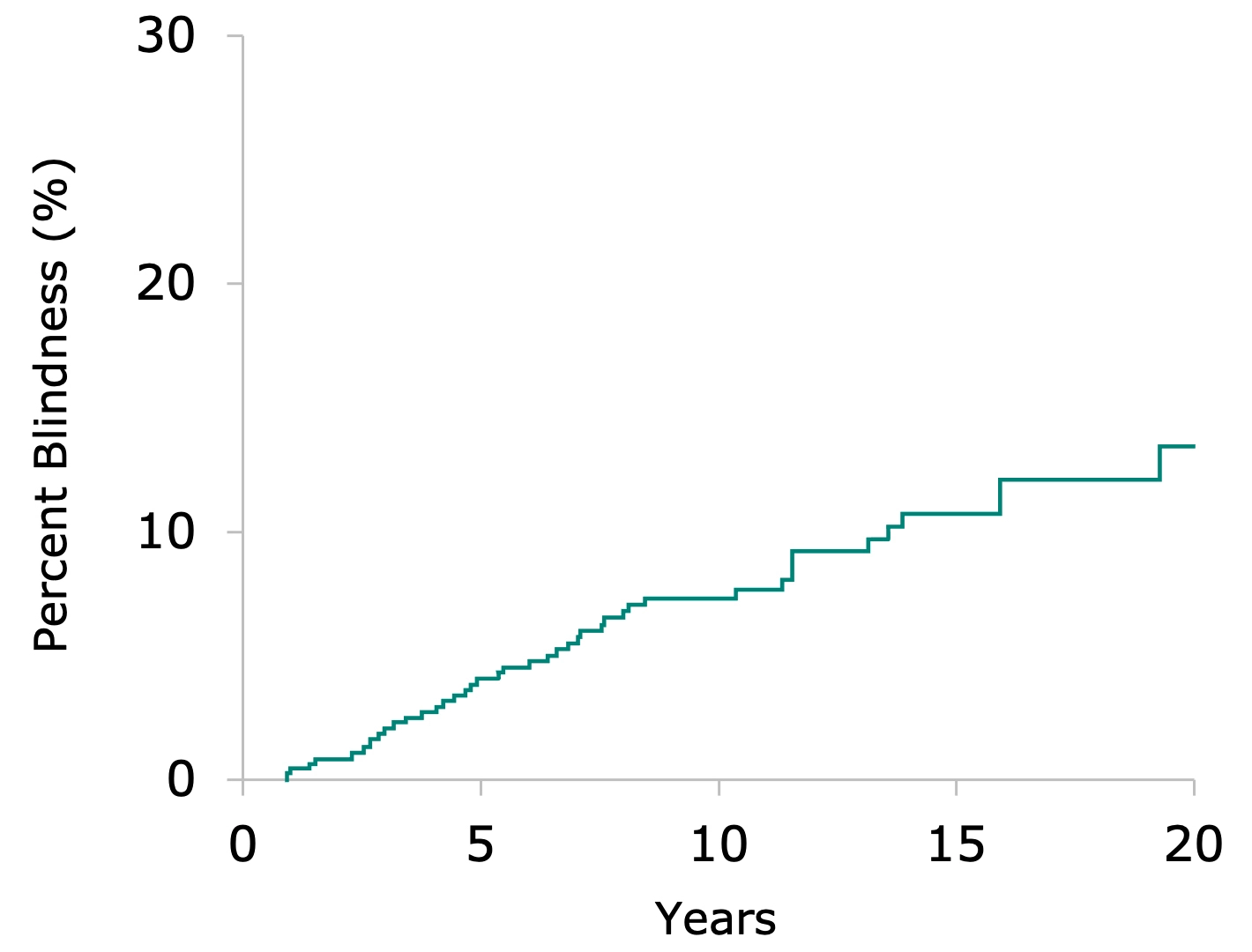
13.5% of patients progressed to blindness
Data collected from 1981-2000
In a 20-year study sponsored by the NIH in Olmsted County, Minnesota2:
Despite being diagnosed and treated with glaucoma drop therapy, the probability of disease progression leading to blindness in at least one eye shown to be 13.5%, and 4.3% bilaterally
Average time to blindness in these patients was 5.8 years
Drops-first management has compounding limitations:
Self-Application of Topical Medication Leads to Adherence Challenges:
of glaucoma patients are non-compliant with glaucoma drop therapy3
of glaucoma patients purposely discontinue glaucoma drop therapy within 6 months
Stepwise Introduction of Additional Topical Therapy has Diminishing Return on IOP Reduction
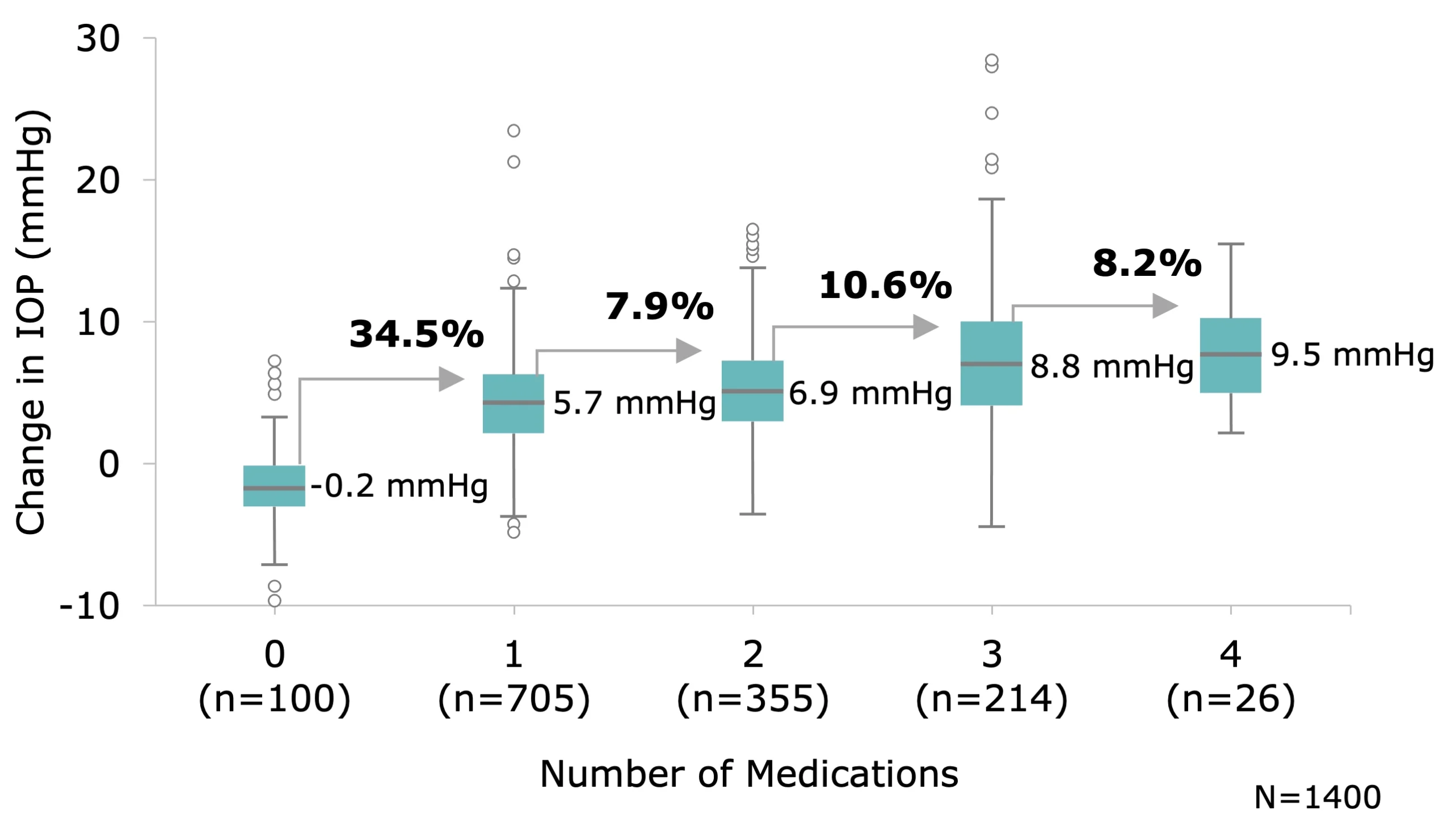
Additional topical medications provide only 8-11% further reduction in intraocular pressure (IOP) following treatment with the first drop.4
Long-term Glaucoma Drop Therapy Leads to Ocular Side Effects
Advancements in Glaucoma Treatment
Advancements in therapeutic options have addressed the limitations of drop-based therapy by effectively lowering IOP without relying on patient self-application of topical pharmaceuticals.1*
-
Lasers
Non-invasive selective laser trabeculoplasty (SLT) to enhance outflow
See Where Lasers Fit in the IG Consensus Protocol -
Procedural Pharmaceuticals
Sustained-release drug delivery systems that provide ongoing IOP control without daily patient action.
See Where Procedural Pharmaceuticals Fit in the IG Consensus Protocol -
Tissue-Sparing MIGS
Minimally invasive procedures that preserve ocular structures
See Where Tissue-Sparing MIGS Fit in the IG Consensus Protocol -
Non-Tissue Sparing MIGS
Procedures that remove or modify tissue to improve aqueous outflow, resulting in more aggressive IOP reduction.
See Where Non-Tissue Sparing MIGS Fit in the IG Consensus Protocol
Interventional Glaucoma: A Proactive Approach
Moving towards a new standard
Incorporating early procedural interventions can slow disease progression more effectively than topical glaucoma drop therapy7
-
Illustration of an eye treated with glaucoma drop therapy -

Illustration of an eye with reduced glaucoma drop therapy
An Interventional Approach that is Advancing Patient Care
Personalize
Personalize glaucoma management by early predictive diagnostics and active monitoring.
Proactive
Proactively manage glaucoma rather than waiting for significant progression or patient non-compliance and adherence.
Effective, low-risk
Effective, low-risk early procedural interventions allows the HCP to re-gain control of glaucoma treatment.
Integrating Interventional Glaucoma Into Your Practice
A consensus protocol was developed by eleven leading ophthalmologists. The Interventional Glaucoma working group reviewed individual treatment strategies and established a consensus-based framework tailored to each stage of disease progression8.
-
Ocular Hypertension
- Lasers
- Procedural Pharmaceuticals
- Tissue-sparing MIGS
- MTMT
-
Mild Glaucoma
- Lasers
- Procedural Pharmaceuticals
- Tissue-sparing MIGS
- Non-tissue-sparing MIGS Procedures
- MTMT
-
Moderate Glaucoma
- Lasers
- Procedural Pharmaceuticals
- Tissue-sparing MIGS
- Non-tissue-sparing MIGS Procedure
- MTMT
- Filtering Surgery
-
Severe Glaucoma:
Favoring a more aggressive intervention, skipping laser to focus on a combined protocol of procedural pharmaceuticals and tissue-sparing MIGS followed by non-tissue-sparing MIGS if IOP is not controlled. Filtering surgery is still the last resort but may be considered when prompt and substantial reduction of IOP is imperative to prevent irreversible optic nerve damage.
- Procedural Pharmaceuticals
- Tissue-sparing MIGS
- Non-tissue-sparing MIGS Procedures
- MTMT and/or Lasers
- Filtering Surgery
-
Bridge therapy: Drops used as a temporary treatment while patient awaits procedural intervention | Supplemental: Drops used in addition to a procedural intervention MTMT: Maximum tolerated medical therapy | Tissue-sparing MIGS: Either trabecular bypass or canaloplasty
Discover the Future of Glaucoma Care
Watch how Glaukos is advancing patient outcomes and supporting practices on their interventional glaucoma journey.
Applying Interventional Glaucoma in Practice
As awareness of interventional glaucoma continues to grow, translating this understanding into clinical practice is essential to advancing patient care.
Clinicians should assess their current treatment protocols and consider where opportunities exist to integrate interventional options earlier in the care continuum.
Discover how Glaukos' products meet the needs of Interventional Glaucoma
iDose® TR (travoprost intracameral implant) 75 mcgiStent Infinite® * Glaukos provided reimbursement for the Interventional Glaucoma Working Group’s time and travel expenses to allow them to participate in consensus-building discussions.
* Glaukos provided reimbursement for the Interventional Glaucoma Working Group’s time and travel expenses to allow them to participate in consensus-building discussions.
"*" indicates required fields
References
- 1. Bedrood S et al. Clin Ophthalmol. 2023;17:3899-3913.
- 2. Malihi M et al. Ophthalmology. 2014;121(1):134-41.
- 3. Nordstrom BL et al. Am J Ophthalmol. 2005;140(4): 598-606.
- 4. Johnson T , Jampel HD. Am J Ophthalmol. 2020;216:110-20.
- 5. Kolko et al. Ocular Surface. 2023;29:456-68.
- 6. O’Hare et al. Clin Exp Ophthalmol. 2012;40(7):675-81.
- 7. Litcher PR et al. Ophthalmol. 2001;108(11):1943-53.
- 8. Funke C et al. Exp Rev Ophthamol. 2025; 20:79-87.
