Corneal Health Solutions
Get There in Time
We’re transforming the standard of care for patients with progressive keratoconus and other corneal ectatic conditions through our commitment to addressing important unmet clinical needs in corneal health.
With inspired innovation, a customer-centric focus, and prolific market access capabilities, we are in the constant pursuit of developing proven solutions in corneal health that empower eye care professionals to deliver optimal care for patients
What are the signs and symptoms of progressive keratoconus, and how can corneal cross-linking help?
Learn More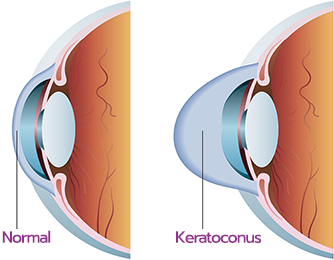
Diagnosing Keratoconus with
iDetect KC
Diagnosing Keratoconus with
iDetect KC
The goal of treatment for iLink® corneal cross-linking patients is to slow or halt the progression of keratoconus. For these patients, continued progression often results in loss of visual acuity or decreased tolerance to contact lens wear, caused by the ongoing changes in the cornea. This means early diagnosis is critical so that progressive keratoconus can be treated sooner.
[Gelles2017/p1/col1/para2/lines6-9; col2/para1/lines5-8; p3/col1/para2/lines1-7]
Bring advanced topography into your practice. Glaukos and Topcon have partnered to offer optometrists the advanced technology of Topcon CA-800 topographer at a lower cost of entry.
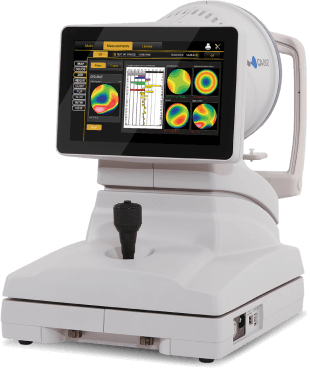
Resources for Your Practice
Our Comprehensive Support. Your Uncompromising Patient Care.
Integrating iLink® into your practice helps you expand care, and provide a wider range of benefits to many of your patients. As a trusted industry leader, we have the experience, tools, and training to make integration easy.


Market Access Support
Looking for Insurance
Reimbursement
Information?

Every patient deserves access to procedures that can help preserve their vision. iLink® corneal cross-linking helps patients avoid sight-threatening disease progression. That’s why the iPath360 program provides strategic and trustworthy market access solutions for all Glaukos procedures and products in glaucoma, corneal health, and retinal disease care.

Explore Patient Resources for Keratoconus Treatment Options
View Patient WebsiteFind a Physician Performing the iLink® Corneal Cross-Linking Procedure
Go to Physician LocatorContact Us Today
to Integrate the iLink®
Procedure Into Your
Practice
Explore additional information about iLink® in our digital brochure and learn how the procedure is transforming the standard of care for progressive keratoconus.
Request
More Info
"*" indicates required fields

